Abstract
Purpose: The purpose of this study was to evaluate the effects of repeated scaling and root planing (SRP), with or without locally-delivered minocycline microspheres (MM) on residual pockets in patients undergoing periodontal maintenance (PMT).
Methods: Patients on PMT were randomized into two groups for treatment of one posterior interproximal inflamed pocket (≥5 mm) with a history of bleeding on probing every 6 months: SRP plus MM (n=30) or exclusively SRP (n=30). Baseline and 24-month measurements included radiographic interproximal alveolar bone height, probing depths (PD), clinical attachment level (CAL), bleeding on probing (BOP), gingival crevicular fluid (GCF), and salivary interleukin (IL) - 1β, (24 month only). Results were analyzed for baseline data or change in measurements after 24 months of treatment between different treatment groups, as well as whether significant changes occurred after 24 months of treatment for each treatment group individually.
Results: Alveolar bone height and GCF IL-1β remained stable over the 24 months. The SRP + MM and SRP groups each demonstrated reduced PD (0.8 ± 0.9 mm and 1.1 ±0.6 mm, respectively, p < 0.001 each), CAL (0.8 ± 0.9 mm and 1.0 ± 0.6 mm, respectively, p < 0.001 each) and BOP (55% and 48%, respectively, p = 0.001 each). However, there were no differences between groups over the 24-month study period.
Conclusion: Scaling and root planning alone, of moderately inflamed periodontal pockets at 6-month intervals, produced stable interproximal alveolar bone height as well as sustained improvements in probing depths, clinical attachment level, bleeding on probing over 24 months; minocycline microspheres were not shown to enhance these results.
- periodontal disease
- periodontal pockets
- non-surgical periodontal therapy
- periodontal maintenance
- scaling and root planning
- chemotherapeutics
Introduction
Dental hygienists in clinical practice often face challenges managing residual inflamed periodontal pockets during periodontal maintenance therapy (PMT). Periodontal pockets with residual or recurrent signs of inflammation during PMT have been shown to be more likely to progress and deserve additional treatment.1 Repeated scaling and root planing (SRP) is often performed in these sites, however, locally applied chemotherapeutics are popular adjuncts to SRP. Minocycline (MM), microencapsulated in a bioabsorbable polyglycolide-co-dl lactide polymer, is commercially available (Arestin®, Orapharma; Bridgewater, NJ, USA) and is inserted into the periodontal pocket in a powder form. Immediately following contact with the gingival crevicular fluid, the polymer begins to hydrolyze and release the minocycline. Administration of MM results in a localized, sustained release of the antibiotic at concentrations of 340 μg per ml into gingival crevicular fluid (GCF) for 14 days, much higher than minimum inhibitory concentrations.2
While MM has been used during PMT since the early 2000's, most studies have investigated clinical outcomes following the application of minocycline microspheres during initial SRP.2-4 The initial investigations demonstrated that SRP plus MM provided greater reductions in probing depth as compared to SRP alone. Application of MM was also shown to reduce measures of inflammation, including interleukin (IL)-1 in gingival crevicular fluid (GCF) over the short term.5
Few studies have evaluated SRP + MM protocols during periodontal maintenance. Meinberg et al.6 studied the difference in clinical parameters between conventional periodontal maintenance and SRP with MM. The authors concluded that SRP and MM resulted in greater PD reduction and few incidences of radiographic bone height loss than in conventional periodontal maintenance. In the Meinberg et al. study, MM was applied at baseline, 1, 3, and 6- month appointments following SRP at the baseline appointment only; control patients received conventional PMT at 3-month intervals for 1 year. 6 An additional study by van Steenberghe et al., evaluated the clinical and microbiological outcomes of repeated application of 2% minocycline ointment subgingivally.7 SRP was performed at baseline, 6, and 12 months; minocycline ointment was applied at baseline, 2 weeks, 1, 3, 6, 9, and 12 months. The authors concluded that repeated application of subgingival minocycline ointment resulted in improvement in both clinical and microbiologic variables over 15 months when compared to SRP alone.
Long-term studies involving the measurement of clinical parameters and inflammatory bio-markers following SRP+MM during conventional PMT protocols are lacking in the literature, yet are important to determine whether adjunctive applications of MM are cost-effective. The purpose of this study was to determine whether repeated application of SRP+MM to a moderately inflamed periodontal pocket at 6-month intervals during PMT would stabilize alveolar bone height, improve clinical parameters, and decrease the level of inflammatory biomarkers when compared with SRP alone a period of 2 years.
Methods
Patient Population and Study Design
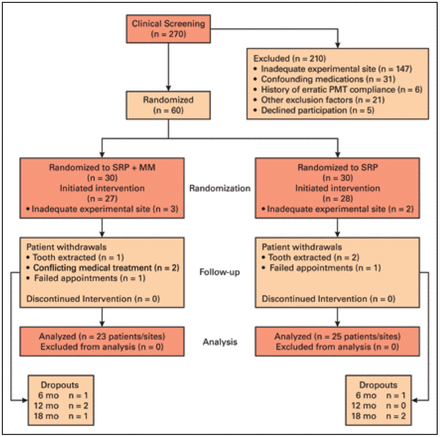
This study received Institutional Review Board approval (IRB #314-12) from the University of Nebraska Medical Center. Patients regularly attending the University of Nebraska Medical Center (UNMC) College of Dentistry for periodontal maintenance therapy were screened for the following inclusion criteria: individuals between 40-85 years of age, a periodontal diagnosis of moderate-severe chronic periodontitis, a history of regular periodontal maintenance therapy (≥2/year), a ≥ 5 mm posterior interproximal pocket with a history of BOP, no systemic diseases (e.g. rheumatoid arthritis, osteoporosis), and not currently taking medications with a significant impact on periodontal inflammation or bone turnover (e.g. chronic nonsteroidal anti-inflammatory drugs, steroids, bisphosphonates, calcitonin, methotrexate, antibiotics). Individuals with tetracycline allergies were also excluded. Patients meeting the inclusion criteria were invited to participate, provided informed consent and then stratified by gender and smoking status. Participants were randomly assigned to test (SRP+MM) or control (SRP) groups by coin toss by a clinician not involved with clinical measurements. (Figure 1).
The same clinician also identified the most posterior ≥5 mm interproximal pocket with a history of BOP as the experimental site. A power analysis was performed for detecting bone loss (primary outcome) at 24 months, based on a previous study of Payne et al.,8 and additional clinical data from maintenance populations in the UNMC clinic. It was assumed that the standard deviation of the change in average bone loss at 24 months was 0.57 mm. The significance level was set at 0.05/2=0.025 based on the Bonferroni method to adjust for two tests conducted under the two treatments separately. If 23-25 subjects per group completed the study it would provide at least 80% power to detect a difference of 0.4 mm (threshold based on two times the standard deviation of replicate measurements)8 in average bone loss at 24 months after treatment in each group at a two-sided significance level of 0.025 via one-sample t test. Therefore, 30 subjects randomized to each group with a 16% drop out rate would yield a sufficient number of participants. Probing depths, CAL, BOP and inflammatory biomarkers in GCF and saliva were secondary outcomes. Saliva collection was added to the protocol after trial commencement and completed at the final (24 month) maintenance appointment.
Randomization flow chart
Full-mouth periodontal maintenance therapy, along with SRP+MM or SRP as well as all repeated measurements at the experimental site were performed at baseline, 6-month, 12-month, 18-month, and 24-month appointments. All clinical, radiographic and laboratory examiners were blinded to the treatment randomization. The study, conducted between October 2012 and October 2015, was also registered with ClinicalTrials.gov (NCT01647282).
Data Collection and Treatment Protocol
A modified radiographic positioning ring (Dentsply-Rinn, Chicago, IL, USA) was used while exposing radiographs at baseline and 24-months, allowing the rectangular radiographic cone to lock into a standardized film-to-source geometry. Measurements were made using digital imaging software (MiPACS Dental Enterprise Solution, Medicor Imaging; Microtek, Hsinchu, Taiwan). Measurements were made from the cementoenamel junction (CEJ) of the test and control sites to the base of the bony defect by two blinded examiners in order to detect interproximal alveolar bone levels (IBL). Measurements were repeated in 10% of samples; intra-class correlation revealed that repeated measurements at baseline was 0.937 (95% CI = 0.538 to 0.993) and at 24 months was 0.983 (95% CI = 0.858 to 0.998), indicating excellent reliability.
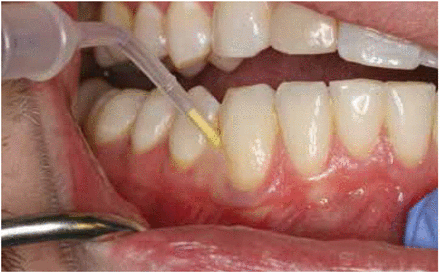
Clinical data were collected at the experimental site by one of two calibrated, blinded periodontists (RR or AK)9 and reported at baseline and 24 months. Data collection at one year has been described previously.9 During data collection, supragingival plaque was removed from the test and control teeth with a dental explorer; if any plaque deposit was visible on the explorer tip after the first pass across the tooth surface it was recorded as “positive.” Following the recording of visible plaque, an absorbent paper strip (Periopaper, ProFLow; Amityville, NY, USA) was inserted into the experimental site sulcus for 30 seconds to collect the GCF sample. Strips contaminated with blood were discarded and a second sample was taken. The paper strip was placed into a coded sterile vial and frozen at -80° C. Gingival recession was then measured at the test and control sites using a University of North Carolina (UNC) 15 probe (Hu-Friedy; Chicago, IL, USA). Probing pocket depths (PD) were then measured at the same site and clinical attachment level (CAL) was calculated. BOP was recorded as positive for sites that bled within 30 seconds. Full-mouth pocket measurements and periodontal maintenance therapy were then completed by the dental student assigned to the case. At the end of the periodontal maintenance therapy appointment, a licensed dental hygienist (JH) performed SRP at the experimental sites and inserted 1 mg of MM into the test site pockets. (Figure 2) Participants returned for 6-month, 12-month, 18-month, and 24-month periodontal maintenance appointments. Because longer recall intervals may increase periodontitis risk,10 the intervals were extended to six months to determine if MM provided more periodontal stability compared as compared to repeated SRP alone in moderate periodontitis patients. At each maintenance appointment, scaling of shallow sites (≤ 4 mm) plus root planing of sites ≥ 5 mm was provided. Saliva collection was accomplished at the 24-month appointment, using a variation of the technique described by Navazesh.11 Patients rinsed with water and expectorated into a sterile collecting tube for five minutes while in a seated position. Saliva samples then were centrifuged at 2,000 RPM for 5 minutes and the supernatant was pipetted into coded sterile vials. Vials were then frozen at -80° C before further testing. Salivary sampling was a protocol change added after the initial informed consent; therefore, all of the subjects re-consented at the beginning of the 24-month appointment.
Application of MM into test site
Analysis of GCF and Salivary Samples
GCF samples from test and control sites and saliva samples were analyzed for IL-1β using quantitative sandwich ELISA kits according to manufacturer's instructions (R&D Systems, Human IL-1β/IL-1F2 Quantikine® ELISA; R&D Systems, Minneapolis, MN, USA). Samples were allowed to thaw at room temperature and GCF strips were placed in 1 ml of phosphate buffer saline and gently agitated for 1 hour during the thawing process. Standard calibration curves were generated. The minimum detectable concentration for the ELISA was 1 pg/ml and the maximum detectable concentration was 262 pg/ml. All samples were analyzed in duplicate. Cytokine levels higher than the maximum detectable level were re-tested; 1:10 dilution. The average of each sample's duplicate was used to determine the total IL-1β, and the total IL-1β was calculated after adjusting for dilutions.
Statistical Analyses
The continuous data at baseline were compared between two treatment groups using two-sample t-test or Wilcoxon rank sum test when the data was normally distributed. Categorical data, at baseline, were compared between groups using a chi-square test. Generalized estimating equations (GEE) with compound symmetry correlation between repeated measures were used to evaluate and compare the treatment effects on different clinical measures separately. The considered clinical measures include continuous measures l (alveolar bone height loss, PD, CAL, GCF or saliva total IL-1β), and categorical measures (presence of BOP or presence of plaque). GEE has been shown to model data without assuming the outcome variable is normally distributed, allowing for the accounting of any correlations between repeated measures at baseline and 24 months on the same subject. The research model contained covariates of treatment (SRP+MM vs SRP), time (24 months versus baseline) and interaction between treatment and time. Identification of link, or logit link were specified for modelling continuous or binary clinical measures over time. A significant interaction between treatment and time indicated significantly different treatment effects on the corresponding clinical measure. Additionally, the time effects for each treatment arm quantified change in the continuous clinical measures or logit scale of the risk of having categorical clinical measure event. Significant time effects under some treatment implied that there was a significant treatment effect on the clinical measure for the corresponding treatment arm. Bonferroni method was used to address multiple comparisons issue. Saliva IL-1β was only available at 24-month appointments. Values were log transformed and compared using two-sample t test between groups. Spearman correlation coefficients and p values testing for non-zero correlation were also calculated between GCF and saliva total IL-1β measurements at baseline or 24 months and the changes in the clinical outcome values at 24 months.
Results
Patient Characteristics
Of the 60 participants randomized for this study, intervention was initiated on 55 subjects due to experimental sites in 5 patients falling below the inclusion criteria of ≥ 5 mm PD at the time the baseline measurements were performed. Forty-eight patients with one experimental site each completed the 24-month study (13% dropout rate). Dropouts were similarly distributed between groups (Figure 1).
All patients were asked to report any symptoms or problems experienced during the study. No adverse events were reported There were no significant differences between the two groups. Patients generally had probing depths ≤ 4 mm except for one 5-7 mm posterior interproximal site with bleeding on probing. Baseline characteristics of patients initiating the study are displayed in Table 1.
Patient Demographics
Radiographic and Clinical Measures
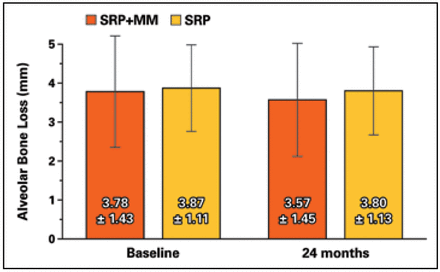
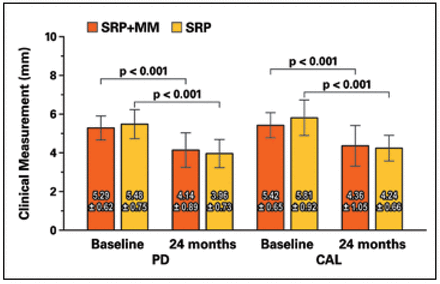
Both groups had stable interproximal alveolar bone height over 24 months at experimental sites with PMT at 6-month intervals. Only one site in each group lost 0.5 mm and no sites lost ≥ 1 mm (Figure 3). Both the SRP+MM and SRP groups each demonstrated significantly reduced PD and CAL from baseline to 24 months (Figure 4). Neither group demonstrated a difference in the amount of plaque accumulation in the experimental site. There were no differences in vertical bone loss between groups at experimental sites.
Inflammatory Measures
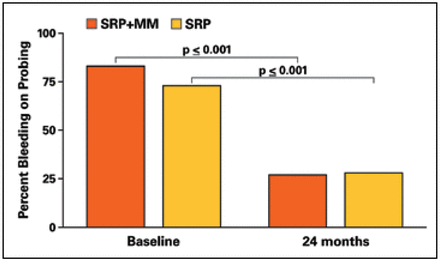
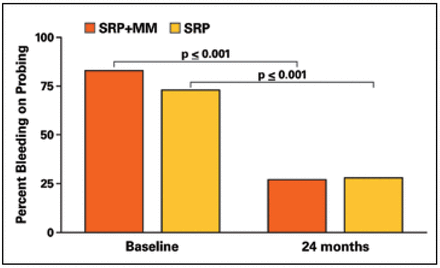
BOP deceased significantly in each group between baseline and 24 months; however, there were no differences between groups (Figure 5). The mean baseline and 24-month measurements of GCF total IL-1β are shown in Figure 6. No differences were noted at baseline or 24 months between groups. In addition, GCF total IL-1β did not change over time.
A significant Spearman correlation was found between GCF total IL-1β at baseline and change in alveolar bone height at 24 months (r=0.34, p=0.017). A concurrent correlation was found between salivary total IL-1β at 24-months and change in PD site over 24 months (r=0.34, p=0.031), and a trend toward a change in CAL site (r=0.31, p=0.055).
Interproximal alveolar bone height from cementoenamel junction at test and control sites
mean ± standard deviation
No significant differences were noted between groups or time points.
Probing depth and clinical attachment loss at test and control sites
mean ± standard deviation
A significant reduction in both measurements was noted between baseline and 24 months for both groups.
No significant differences noted between groups.
Percent bleeding on probing at test and control sites
Log transformed IL-1β in test and control site gingival crevicular fluid or saliva
mean ± standard deviation
No significant differences were noted between groups or time points.
Discussion
The aim of this study was to determine the effect of repeated MM when used in conjunction with SRP in patients receiving regular periodontal maintenance therapy (6-month intervals) compared to performing SRP alone during periodontal maintenance. All patients had received periodontal treatment and regular periodontal maintenance therapy prior to enrolling in the study. Each patient was analyzed at one posterior pocket ≥5 mm with a history of BOP.
The primary outcome measure in this study was change in interproximal alveolar bone height. Neither group demonstrated a significant change from baseline to 24 months even though the study was powered to detect changes of 0.4 mm mean bone loss. Results showed that only one site in each group lost 0.5 mm alveolar bone height over the duration of the 24-month study. Payne et al.12 reported that in post-menopausal women receiving sub-antimicrobial doses of doxycycline or placebo and 3 to 4-month periodontal maintenance therapy, alveolar bone height remained stable in both groups. At the end of 2 years, approximately 90% of sites showed no significant change (≤0.4 mm) in alveolar bone height, based on two times the standard deviation of replicate measurements. In the current study with periodontal maintenance therapy performed every 6 months, 96% of subjects showed no change in alveolar bone height over 24 months. Results in the present study indicate the stability of the alveolar bone height at the experimental sites, is presumably supported by repeated SRP.
When evaluating the baseline and 24-month clinical and inflammatory measurements, no significant differences were found between the two groups. However, both groups experienced a statistically significant decrease in PD, CAL, and BOP. No changes were found from baseline to 24 months in IL-1β levels in either group. Meinberg et al.6 reported improvements in PD of 0.9 ± 0.1 mm in their SRP+MM group at one year compared with 0.4 ± 0.1 mm in the conventional periodontal maintenance group. CAL was not reported. Meinberg et al. also administered 4 doses of MM over a 6-month period with only one session of SRP in contrast to the current study which administered 4 doses of MM, in combination with SRP at each session, every 6 months for 2 years.
The current study protocol may better approximate a more feasible situation clinically. In this study, PD reductions of 0.8 ± 0.9 mm in the SRP+MM and 1.0 ± 0.6 mm in the SRP groups were shown at 24 months. Performing SRP every 6 months appeared to promote greater PD reduction over two years than a single episode of SRP at one year. When evaluating recently-published, one-year data from this clinical trial,9 the PDs were shown to numerically reduced further, although not statistically significant, at 24 months following the initial improvements at 6 months. These outcomes also are consistent with previous reports showing that a 1 mm decrease in PD can be expected following acceptable SRP without adjunctive therapy, one year post-initial treatment.13
When considering CAL, the current study demonstrated significant gains in CAL in both groups with the SRP+MM group gaining 0.8± 0.9 mm and the SRP group gaining 1.0 ± 0.7 mm at the conclusion of 24 months. This CAL improvement aligns with previous data regarding post-treatment responses to SRP.13
Results from this study showed that both the SRP+MM and SRP groups demonstrated significant decreases in the incidence of BOP but continued to have high levels of explorer-detectable plaque. The high plaque levels may be due to focusing exclusively on posterior interproximal sites and using a very sensitive positive threshold (any deposit visible on the explorer tip after the first pass across the tooth surface). The SRP+MM group showed that 59% of subjects with BOP at baseline did not have BOP 2 years later and the SRP group showed 52% subjects with BOP at baseline did not exhibit BOP 2 years later. This reduction in BOP following therapy is consistent with previous findings.13 While the presence of BOP is not a reliable predictor of disease activity, reduction and elimination of BOP may be used as a criterion for stability.14 Considering BOP as an indicator for periodontal stability, results from this study would reinforce the concept that periodic SRP can lead to long-term reduction in BOP, thus periodontal stability, regardless of the addition of MM and, in spite of persistent supragingival plaque. Miyamoto et al.15 also showed that patients who were compliant with periodontal maintenance therapy demonstrated a greater decrease in BOP levels (to 38%) when compared to patients with poor compliance (43%).
Periodontal disease and disease severity have been associated with GCF IL-1β levels.16 Neither group in our study experienced significant changes from baseline to 24 months in total GCF IL-1β. Previous studies, following patients at 6 and 24 weeks respectively, report that SRP produces a reduction in GCF IL-1β at various time periods.16 Additionally, these studies followed patients after initial SRP rather than patients in ongoing periodontal maintenance therapy. Current findings from this study suggest that baseline IL-1β levels were already lowered by previous periodontal maintenance therapy and that BOP may be a simpler and more sensitive measure of local inflammation than measures of GCF IL-1β.
Salivary levels of IL-1β have been shown to reflect periodontal disease severity.17-19 In the current study, total IL-1β was measured at the 24-month periodontal maintenance therapy appointment. Results were similar between the SRP+MM group (13.2 ± 1.2 pg, log transformed) and SRP group (12.8 ± 1.2 pg, log transformed). Similar to GCF IL-1β, evidence shows that periodontal therapy may reduce the amount of IL-1β in saliva.18 These findings again suggest that salivary IL-1β at 24 months may have already been lowered by previous periodontal therapy.
Current smokers were included in both the SRP+MM group (n=8) and SRP group (n=4). Tobacco use has been shown to affect the severity of periodontitis and the individual's response to therapy. Cigarette smoking has been shown to be associated with a 2-8 times increased risk for CAL and alveolar bone loss.20 Bergstrom21 found that over a 10-year period, smokers lost more periodontal bone height (0.74 ± 0.59 mm) than non-smokers (0.27 ± 0.29 mm). Labriola et al.22 found that PD ≥ 5 mm were reduced more in non-smokers when compared to smokers during SRP by an average of 0.433 mm. Previous data published from the current study found no difference between the clinical outcomes of smokers and non-smokers at one year, as was the case with the current results at two years (data not shown).9
There are several limitations to this study. Study participants were already receiving regular periodontal maintenance therapy and were considered to be compliant patients and periodontally stable. Perhaps, different findings would have been observed in a population with evidence of progressive periodontitis at the baseline visit. In addition, the majority of the experimental sites were of moderate depth (5 mm and 6 mm), and the use of MM in deeper pockets may be more effective. However, it has been demonstrated that deeper pockets are reduced more effectively with flap surgery.23 Several patients also dropped out of the study for various reasons. Since the primary outcome, interproximal bone loss, had only two time points (baseline and 24 months), traditional intent-to-treat analyses were not straightforward. However, dropouts were similar between groups and the remaining patient numbers retained adequate power.
Results obtained from this study would encourage a more judicious use of MM in periodontal maintenance patients. Perhaps the use of MM would be most cost-effective and clinically-relevant in periodontal maintenance patients with deep, inflamed periodontal pockets but who either refuse or are unable to have periodontal surgery. Further study could enlighten oral health care providers on the most appropriate use of this drug within the periodontal maintenance population.
Conclusion
The small-sample size of the current study does not allow for the conclusion that scaling and root planning and minocycline microspheres and scaling and root planing alone are equivalent therapies. Repeated scaling and root planing alone of inflamed moderate periodontal pockets, at 6-month intervals, produced stable interproximal alveolar bone height as well as a long-term improvement in bleeding on probing, probing depths, and clinical attachment levels over 24 months. Repeated application of minocycline microspheres was not found to enhance scaling and root planing results.
Acknowledgements
The authors thank Marian Schmid for technical assistance with ELISAs, Deb Dalton for the manuscript preparation, and Drs. Jeffrey Payne and Henry St. Germain for their critical review of the manuscript. Kim Theesen is much appreciated for assisting with the graphic design.
Primary funding for this study was provided by the Dr. D.H. Reinhardt Scholar Program, of which, Dr. Amy C. Killeen is the 2012-2016 D.H. Reinhardt Scholar. Dr. Richard Reinhardt, the secondary investigator in this study, is the grandson of the late Dr. D.H. Reinhardt. Neither Dr. Richard Reinhardt nor his family has any oversight over the awarding of these scholar program funds. Additional funding was provided by the late Dr. Mick Dragoo and his wife Mary, and the Nebraska Dental Association Foundation. None of the authors declare any conflicts of interest.
Footnotes
Amy C. Killeen, DDS, MS is an assistant professor; Jennifer A. Harn, RDH, BS is an assistant professor; both in the Department of Surgical Specialties, Section of Periodontics, University of Nebraska Medical Center, Lincoln, NE.
Jeffrey Jensen, DDS, MS and Shawn Custer, DDS are graduates of the College of Dentistry, University of Nebraska Medical Center, Lincoln, NE.
Fang Yu, PhD is a professor in the Department of Biostatistics, University of Nebraska Medical Center College of Public Health, Omaha, NE.
Richard A. Reinhardt, DDS, PhD is a professor and co-director of undergraduate periodontics, Department of Surgical Specialties, Section of Periodontics, University of Nebraska Medical Center, Lincoln, NE.
This manuscript supports the NDHRA priority area: Client level: Basic science (Diagnostic testing and assessments).
- Received June 14, 2017.
- Accepted April 19, 2018.
- Copyright © 2018 The American Dental Hygienists’ Association