Abstract
Purpose: Diabetes and periodontal disease are conditions considered to be biologically linked. Prediabetes is a condition in which individuals have blood glucose levels, impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) or glycated hemoglobin (A1C) levels higher than normal but not high enough to be classified as diabetes. Few human studies address the relationship between periodontitis and prediabetes or clarify an association between periodontitis and prediabetes. The purpose of this pilot study was to examine the impact of non-surgical periodontal therapy (NSPT) on clinical measures of glycemic control in prediabetes.
Methods: Prediabetes measures of IFG, IGT, A1C and periodontal measures of pocket depth (PD), clinical attachment level (CAL), plaque index (PI) and gingival index (GI) were taken at baseline and 3 months in 12 subjects with prediabetes and chronic slight to moderate periodontitis. Blood samples were taken from each subject following an 8 hour fast. This study controlled for changes in medications, body-mass index, physical activity and diet.
Results: Comparison of mean prediabetes and periodontal measures from baseline and post-treatment at 3 months demonstrated clinical improvement for both periodontal and prediabetes measures. A mean reduction in PD of 0.27 (p=0.003), CAL of 0.32 (p=0.050) and A1C of 0.19 (p=0.015) reached statistical significance.
Conclusion: This pilot study suggests NSPT improves A1C and periodontal measures at 3 months. The robustness of measures is limited due to the small sample size and lack of a control group. Further larger scale studies using a randomized control design would be informative.
- diabetes mellitus
- prediabetes
- impaired fasting glucose
- impaired glucose tolerance
- glycated hemoglobin
- A1C
Introduction
In 2010, U.S., individuals aged 65 years and older (10.9 million, 26.9%) had diabetes.1 The number of people with diabetes is projected to increase from 171 million in 2000 to 366 million in 2030, and is attributed to type 2 diabetes mellitus (T2DM).2 T2DM can range from insulin resistance with relative insulin deficiency to an insulin secretory defect with insulin resistance accounting for 90 to 95% of cases.1 The diagnosis of diabetes is based on fasting plasma glucose (FPG) (FPG≥126 mg/dl (7.0 mmol/l), 2 hour plasma glucose level of ≥200 mg/dl (11.1 mmol/l)) or glycated hemoglobin (HbA1C or A1C) of ≥6.5%.3 The condition of prediabetes may precede diabetes and is a condition where individuals have impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) or A1C levels higher than the normal range, but not high enough to be classified as diabetes.3 People with prediabetes are at a higher risk for developing T2DM.3 In 2005 to 2008, based on fasting glucose or A1C levels, 35% of the U.S population aged 20 years or older and 50% of those aged 65 years or older had prediabetes.3
Complications of diabetes can lead to heart disease, stroke, hypertension and susceptibility to other diseases.1 Individuals with diabetes are at increased risk for chronic infections and inflammation of the oral tissues, including periodontal disease, dental caries and oral candidiasis.4,5
Diabetes and Periodontal Disease
Periodontal disease is a chronic multifactorial infectious disease of the supporting tissues of the teeth with inflammation and destruction of the underlying supporting tissues. Based on the NHANES III data (1988 to 1994), it is estimated approximately half of the U.S. population ≥30 years have periodontal disease.6,7
Some studies have suggested a bidirectional relationship between glycemic control of patients with diabetes and treatment of periodontal disease.8 A systematic review for glycemic control and periodontal disease found a statistically significant reduction of -0.40% in A1C for scaling/root planing and oral hygiene (+/- antibiotic therapy) versus no treatment/usual treatment after 3 to 4 months.8 In general, every percentage point drop in A1C blood test results (e.g., from 8 to 7%) can reduce the risk of microvascular complications (eye, kidney and nerve diseases) by 40%.1
Periodontal disease is a complex inflammatory disease initiated by oral microbial biofilm with complex interactions between the plaque biofilm and host immune inflammatory response. The inflammatory response results in alterations in bone and connective tissue homeostasis.9-11
There is evidence to suggest a link between periodontitis and several systemic diseases, among which atherosclerosis and T2DM may have the strongest evidence.12 These periodontitis-linked systemic diseases may be caused by an oral–hematogenous-spread organisms passively transported in the blood vessels to distant sites of the body where they penetrate the vessel wall of oral bacteria.13 Amongst the 400 species of subgingival plaque organisms, porphyromonas gingivalis, a gram negative microorganism, is implicated as a major causal species in the initiation and progression of periodontal disease, and induces a local chronic host inflammatory response resulting in bone destruction.14,15
The local inflammation leads to a chronic level of systemic inflammation characterized by elevated plasma levels of inflammatory mediators, such as TNF-alpha, Interleukin-6 IL-6 and acute phase proteins, such as C-reactive protein (CRP).16 An accumulating body of evidence suggests inflammation may play a crucial intermediary role in pathogenesis of T2DM, thereby linking diabetes with a number of commonly coexisting conditions thought to originate through inflammatory mechanisms. In this regard, substantial experimental evidence and more recent cross-sectional data suggest IL-6 and CRP, markers of subclinical systemic inflammation, are associated with hyperglycemia, insulin resistance and overt T2DM.17-26
Prediabetes and Periodontal Disease
Prediabetes generally refers to an intermediate stage between normal glucose levels and the clinical measures of T2DM, encompassing both IFG and IGT. As defined by the American Diabetes Association (ADA), prediabetes is a fasting plasma glucose (FPG) of at least 100 mg/dl (5.6 mmol/liter) but less than 126 mg/dl (7.0 mmol/liter), which is frequently termed IFG, or an abnormal 2 hour response to a 75 g oral glucose tolerance test (OGTT) of at least 140 mg/dl (7.8 mmol/liter) and less than 200 mg/dl (11.1 mmol/liter), which is often termed IGT.3 According to the ADA guidelines, a glycated hemoglobin (HbA1c or A1C) of 5.7 to 6.4% is diagnostic for prediabetes.27 Individuals with IFG and/or IGT are at relatively high risk for the development of diabetes and cardiovascular disease. The prediabetic state can be an intermediary stage for obesity, dyslipidemia with high triglycerides and/or low HDL cholesterol, hypertension and microvasular complications of diabetes.3 A meta-analysis of 156 studies conducted by the Agency for Healthcare Research and Quality showed that a person with prediabetes was 5 to 15 times more likely to develop T2DM than those without the condition.28
Chronic inflammation and dysfunction of cells lining blood vessels exists in individuals with prediabetes, especially in the IFG and IGT populations.29 The Insulin Resistance Atherosclerosis Study hypothesized insulin sensitivity may be related to inflammation in non-diabetic subjects, with the results finding strong and independent associations of elevations in inflammatory markers, namely CRP with high insulin resistance.21
There are animal studies addressing the relationship between periodontitis and prediabetes.30 The Zucker Fatty Rat (ZFR) is a recognized model of prediabetes, characterized by hyperinsulinemia, dyslipidemia and moderate hypertension.30 Female ZFRs develop T2DM after consuming a high fat diet, which makes them excellent models to investigate the effect of periodontitis for prediabetes and the onset of T2DM in obese humans.31 Animal studies examining whether periodontitis affected the prediabetic state found prediabetes worsened with periodontal disease and was associated with deterioration of glucose metabolism in ZFRs, suggestive of a progression toward diabetes. Periodontal disease also affected glucose regulation in lean rats.31
A review of the literature exploring the relationship of periodontal disease and measures of prediabetes was elusive. Two Japanese epidemiological studies explored the relationship between periodontal disease and IGT, and found no significant difference in individuals with IGT and levels of periodontal disease.32,33 Alternatively, 2 Japanese prospective and cross-sectional studies indicated IGT may be a risk factor for periodontal disease.34,35 Another Japanese cross-sectional study found a relationship between periodontal status and A1C in a non-diabetic population while it did not reach statistical significance.36
In a case-control study to determine if glycosylated hemoglobin was elevated in patients with periodontitis who had not been diagnosed with diabetes, periodontitis was associated with a slight elevation in A1C.37 Several limitations were observed in the study, such as use of a Point-Of-Care (POC) instrument to measure A1C instead of a blood draw and standard laboratory test, and no controls for confounders, such as changes in physical activity, weight or diet.
A cross-sectional study conducted in Israel found higher alveolar bone loss was associated with fasting glucose level. A higher prevalence of alveolar bone loss was found amongst non-diabetic males with a fasting glucose level of ≥100 mg/dL than among individuals with <100 mg/dL, suggesting fasting glucose as a predictor for future T2DM, or a possible role in glucose imbalance and T2DM development.38
In a prospective study of a German, non-diabetic population with periodontal disease compared to periodontally healthy participants, those with periodontal disease had 0.08% greater increase in A1C after 5 years.39 There was a positive association between periodontal status and 5 year A1C changes.39
In a cross-sectional study, chronic periodontitis measured by CAL and PD was positively associated with IFG and DM in U.S. adults after adjusting for confounders. An obvious limitation with this cross-sectional study is the determination of whether IFG led to periodontitis or, alternatively, periodontitis led to IFG.40
This review revealed a gap in the literature for randomized control trials to study the relationship between periodontal disease and prediabetes. The purpose of this pilot study was to determine the impact of NSPT on clinical measures of prediabetes and periodontitis.
Methods and Materials
This was a quasi-experimental design of individuals previously diagnosed with prediabetes and chronic periodontitis. It took place in the Forsyth School of Dental Hygiene clinic located in Boston.
The study was approved by the Massachusetts College of Pharmacy and Health Science Institutional Review Board. All participants provided informed consent and received a Forsyth School of Dental Hygiene Health Insurance Portability and Accountability Act (HIPPA) form. Participants were recruited from flyers distributed in local health care facilities, a poster displayed in the clinic, and an ad placed in a local daily paper. Periodontal therapy was provided at the same study site and performed by the same registered dental hygienist to ensure consistency in patient care. Participants included 5 females and 7 males. The age range of participants was 35 to 75. Risk factors for diabetes included level of physical activity, waist circumference, weight, height and diet, and were assessed at baseline and at 3 months.
This study measured IFG, IGT, A1C and periodontal parameters PD, PI, GI and CAL for improved clinical measures of prediabetes at 3 months post-NSPT in subjects with prediabetes and treated chronic slight to moderate periodontitis. Periodontal measurements were performed by a single examiner and registered dental hygienist. The examiner was calibrated for reproducibility of PD and CAL measurements by conducting a periodontal examination on random quadrants in 10 volunteers. Duplicate measurements were performed with follow-up repeated measures within 1 week to provide intra-examiner reliability information. Intra-rater reliability was established at 99% agreement ±1 mm in 10 subjects.
A single registered dental hygienist administered NSPT using the American Academy of Periodontics parameter on chronic periodontitis with slight to moderate loss of periodontal support and oral hygiene instructions at the study site.41 Participants were asked to perform daily oral hygiene, including interdental care and tooth brushing.
The participant criteria consisted of:
≥21 years of age
Understand written and spoken English and able to provide informed consent
Previously diagnosed with prediabetes
Under the care of a primary care physician
Having periodontal disease confirmed by periodontal examination revealing proximal attachment loss of ≥4 mm and in >2 non-adjacent teeth
Dentate (minimum of 16 natural teeth with at least 2 molar proximal contacts)
No periodontal therapy in the past 6 months
Individuals were excluded based on the following criteria:
Previous diagnosis of type 1 or type 2 diabetes
Use of medications to prevent diabetes
Tobacco use in the past year
Blood dyscrasias, such as hemophilia
Use of anticoagulants, such as warfarin, immunocompromised or taking medications leading to compromised immunity
Requiring prophylactic antibiotics for dental care as defined by 2008 American Heart Association (AHA) guidelines
Currently pregnant, planning pregnancy prior to study end, <3 months postpartum, or breastfeeding
Unable or unwilling to complete the OGTT or fingerstick
In need of emergent medical consultation or dental treament
The OGTT requires consumption of a glucose-containing liquid. A fasting glucose blood test was performed on all participants after an 8 hour fast. After the finger stick, participants were asked to drink 75 milligrams of Trutol® (Thermo Fisher Scientific, East Providence, RI) and to have a second finger stick 1 hour and then 2 hours (±15 minutes) after the first. A finger stick was used to gather a blood sample from each participant at the baseline, and 1 and 2 hour time points. A POC glucometer used in hospitals, StatStrip™ Glucose Hospital Meter (Nova Biomedical Corporation, Waltham, Mass.), with a 95 to 97% correlation to plasma blood glucose levels was used.42 Finger sticks were done by trained study personnel at the baseline, and 1 and 2 hour time points during the initial examination and at the 3 month examination.
A1C was measured by a POC instrument at baseline and 3 months. The DCA Vantage™ (Siemens Healthcare, Erlangen, Germany) found in an investigation of the conformance with the National Glycohemoglobin Standardization Program certification criteria of various HbA1C instruments to meet the acceptance criteria of having <3% imprecision, which makes it equivalent to laboratory-based methods.43,44
A periodontal chart including PD, CAL, GI, PI and number of missing teeth were recorded at baseline and 3 months. PD and CAL recordings were made using a UNC-12 periodontal probe on 6 surfaces of all teeth except for third molars. PD was measured as the distance from the free gingival margin to the base of the periodontal pocket. CAL was measured as the distance from the cemento-enamel junction to the base of the sulcus or periodontal pocket (CAL=PD - (CEJ – gingival margin (GM)). Where the GM was subject to recession and the CEJ was exposed, the distance from the CEJ to the GM was given a negative value. Where the GM covered the CEJ, the distance between the GM and CEJ was given a positive value.
The GI was evaluated using a mouth mirror and a UNC-12 probe to determine changes in color, texture, tendency to hemorrhage and presence or absence of ulceration. The gingiva around each tooth was divided into 4 areas corresponding to the mesial (M), distal (D), buccal (B) and lingual (L) surfaces of the tooth, and each of the 4 areas around each tooth was given a score of 0 to 3. The PI evaluated the amount of plaque and soft debris at the gingival margins of the teeth and the 4 gingival areas of each tooth were B, L, M and D and were given scores ranging from 0 to 3.
Height and weight were measured by study personnel. A single scale was used for all participants at baseline and 3 months. The scale was placed on a hard, flat surface and checked for zero balance before each measurement. Body Mass Index (BMI) was calculated using a formula: BMI=(weight (lbs.)*703)/height squared (inches2).
The waist circumference was measured by placing a tape measure around the abdomen just above the hip bone. The tape was snug but not compressed on the skin and was parallel to the floor. Participants were asked to relax, exhale and then the measure was taken.
The NHANES Food Frequency Questionnaire was used to assess dietary habits at baseline and 3 months for changes in the macronutrients, i.e. carbohydrates, fat and protein may impact glycemic control and possibly IGT. Changes in physical activity can also impact IGT and therefore the physical activity inventory from the Behavior Risk Factor Surveillance System was utilized to control for this factor.
Data was collected for measures of periodontal disease and prediabetes. The mean for each measure was analyzed by the nonparametric Wilcoxon signed rank test for paired data with statistical significance defined at p<0.05 using SPSS Statistical Software 17.0.
Results
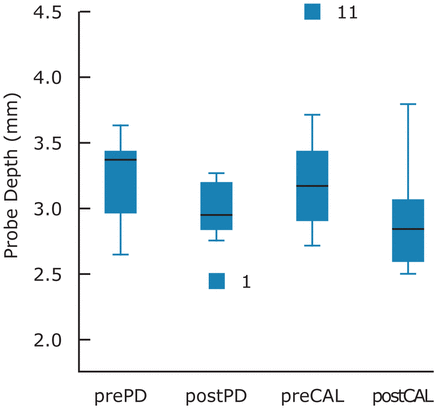
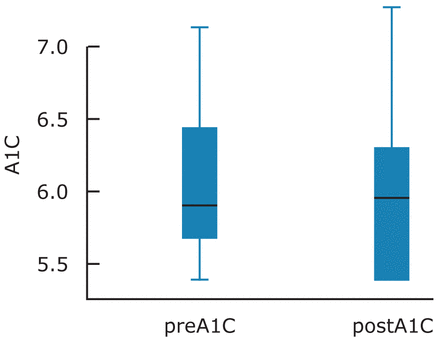
The study was completed by 12 patients. The prediabetes measures of A1C, IFG and IGT at 1 hour and 2 hours, as well as the periodontal measures of GI and PI, were compared between the 2 time intervals. PD and CAL were analyzed as the mean whole mouth measures and also isolating number of sites ≥4 mm per individual at baseline and at 3 months. Comparison of mean prediabetes and periodontal measures from baseline and post treatment at 3 months demonstrated an improvement in both clinical measures of prediabetes and periodontal disease (Tables I, II). The measures of IFG, IGT, PI and GI did not reach statistical significance. However, improvements were noted between the baseline and 3 months for all measures. There was a statistically significant difference between the baseline and 3 month measures of A1C (p=0.02), overall PD and ≥4 mm PD (p=0.003, p=0.055) and overall CAL and ≥4 mm CAL (p=0.050, 0.005) (Figures 1, 2). Weight was compared at baseline and at 3 months with a mean increase of 0.05%.
Discussion
In this pilot study, individuals with prediabetes and periodontal disease received NSPT to determine whether there were clinical differences between the baseline clinical prediabetes and periodontal measures and the 3 month measures. Improvement was observed between baseline and 3 months for both prediabetes and periodontal measures. No significant changes in medications, BMI, physical activity or diet were noted.
The results showed a statistically significant difference in the A1C (-3%, p= 0.02) from baseline and 3 months. These results are similar to previous studies suggesting that periodontal disease may be associated with elevated blood glucose levels in individuals without DM.34-39,45 Providing NSPT was impactful on the measures for PD and prediabetes for the study participants. Reducing A1C by any degree is beneficial and providing treatment of periodontal disease may lessen the risk for developing DM.
Periodontal Measures at Baseline and 3 Months
Prediabetes Measures at Baseline and 3 Months
Means, Standard Deviation and Standard Error at Baseline and Post-Treatment for PD and CAL
Limitations of this study were the small sample size, lack of randomization and a control group which was reflected in the study design. Recruitment of qualified participants was challenging. An affiliation with a medical center where individuals diagnosed with prediabetes could be referred would have been advantageous.
Blood glucose measures were conducted using a POC glucometer with a 95 to 97% correlation to plasma blood glucose levels and a POC HbA1C instrument that met the acceptance criteria of having <3% imprecision. Regardless, the POC tests cannot be substituted for a laboratory test. Advantages to using these tests were convenience, ease of use and patient compliance.
Studies suggest inflammation as a common denominator in periodontal disease, diabetes, prediabetes and other systemic diseases. However, the mechanisms between inflammation and these diseases are not fully understood. Because periodontal disease is an inflammatory disease, further larger scaled, randomized controlled trials controlling for confounders are needed to demonstrate its effect on blood glucose at a metabolic level. Studies should focus on prevention at the earliest stage possible. In addition, oral health should be included in diabetes prevention and management programs.
Conclusion
These findings suggest treating periodontal disease with NSPT reduced A1C levels in prediabetic individuals. Treating periodontal disease had a positive impact on at risk prediabetic individuals and reduced their overall blood sugar glucose. This is important because the incidence and prevalence of diabetes and prediabetes is increasingly becoming a global health concern. Primary care providers are trying to address the diagnosis of diabetes and its complications; however, many health care providers and patients fail to make the oral health connection.
Means, Standard Deviation and Standard Error at Baseline and 3 Months Post-Treatment for A1C
Acknowledgments
The authors thank ADHA, IOH for funding of this grant, J&S Medical Associates – a division JRB Medical Associates, Framingham, Mass., for use of the DCA Vantage.
Footnotes
-
Lori Giblin, RDH, MS, is an Assistant Professor, Forsyth School of Dental Hygiene MCPHS University. Linda Boyd, RDH, RD, EdD, is Dean of the Forsyth School of Dental Hygiene MCPHS University. Lori Rainchuso RDH, MS, is an Assistant Professor, Forsyth School of Dental Hygiene MCPHS University. Dianne Smallidge, RDH, BS, MDH, is an Assistant Professor, Forsyth School of Dental Hygiene MCPHS University.
-
This study supports the NDHRA priority area, Clinical Dental Hygiene Care: Investigate the links between oral and systemic health.
-
This project won 1st place in the ADHA Sigma Phi Alpha Journalism Award Competition, June 2013, under the master or doctoral level category. Award provided by a generous grant from Johnson & Johnson Healthcare Products, Division of McNEIL PPC, Inc.
- Copyright © 2014 The American Dental Hygienists’ Association