Abstract
Purpose: Various mechanical and chemotherapeutic methods are used to control dental plaque accumulation and prevent or reduce gingivitis. The purpose of this 12-week clinical trial was to investigate the effects of various combinations of supervised mechanical and chemotherapeutic regimens on the prevention and reduction of plaque, gingivitis, and gingival bleeding.
Methods: Volunteers presenting with some evidence of gingivitis and no severe periodontitis were randomized into four groups: brush only (BO); brush/rinse (BR); brush/floss (BF); brush/floss/rinse (BFR) for this examiner-blinded clinical trial. Toothbrush, toothpaste, floss and a mouthrinse containing a fixed combination of four essential oils (EO) and training/instructions were provided to participants as per their assigned group. Participants performed their regimen at home, under virtual supervision, once each weekday; the second daily and weekend uses were unsupervised. Assessments included oral hard and soft tissue, plaque, gingivitis, and gingival bleeding (weeks 4, 12); probing depth and bleeding on probing (week 12).
Results: Of 213 enrolled participants, 209 completed the study. After 12 weeks, plaque, gingivitis, and gingival bleeding were significantly reduced in groups BR (35.8%, 50.8%, and 71.0% respectively, p<0.001) and BFR (32.8%, 54.1%, and 78.2% respectively, p<0.001) compared to BO. After 12 weeks, gingivitis and gingival bleeding were significantly reduced in the BF group (9.2%, p=0.013 and 17.5%, p=0.003, respectively), however there were no significant reductions in plaque in the BF group as compared to the BO group (p=0.935).
Conclusions: Oral care regimens that included a mouthrinse containing a fixed combination of four EOs (BR and BFR), demonstrated statistically significantly reduced plaque, gingivitis, and gingival bleeding as compared to BO and BF after 12 weeks. The BF regimen statistically significantly reduced gingivitis and gingival bleeding but did not statistically significantly reduce plaque compared to BO after 12 weeks.
Introduction
Dental biofilm (plaque) is a primary etiologic factor in the two most widely prevalent dental diseases, caries and gingivitis, and is regarded as an underlying cause of gingival inflammation.1 A variety of mechanical methods including toothbrushing, flossing and the use of other interdental cleaning devices are recommended for controlling the accumulation of plaque biofilm. Dental floss is classified by the Food and Drug Administration as a Class I medical device for removal of plaque and food particles between teeth to reduce tooth decay.2 Historically, the use of a silk thread for interdental cleaning was first documented by a dental surgeon in the early 1800’s.3 While the materials used to manufacture dental floss have advanced significantly, patient adoption of flossing as a regular component of an oral hygiene regimen has not conformed to professional recommendations.
In a cross-sectional study using the National Health and Nutrition Examination Survey (NHANES) 2011-2014 data, 35% of participating adults (n=6939) reported having used dental floss, or any other interdental cleaning device, no more than once in the previous seven days.4 Results from the nationwide NHANES study reflect that compliance with commonly recommended oral hygiene regimens for interdental cleaning is low.4 In a systematic review of the home use of interdental cleaning devices on preventing and controlling periodontal diseases and caries, Worthington and colleagues reported low certainty of evidence for flossing to reduce gingivitis over one to six-month time frames.5 Studies examining the proportion of bleeding sites and plaque were found to be inconsistent in the review, leading to a very low certainty of evidence for the benefits of flossing and these clinical outcomes.5 Worthington et al. also discussed a study showing that individuals have difficulty mastering flossing techniques and lack the motivation to do so.5
Chemotherapeutic agents, such as various toothpastes and mouthrinses, provide an additional means to control plaque and reduce gingivitis. Adjunctive chemotherapeutic agents have been studied extensively and numerous systematic reviews have been published. In one systematic review and meta-analysis of the efficacy of these agents in managing gingivitis, Serrano et al. found that toothpaste and mouthrinse formulations with specific plaque control agents provided significant improvements in oral health outcomes as measured by plaque and gingivitis indices, including gingival bleeding.6 Mouthrinses containing a fixed combination of four essential oils (EO) (LISTERINE® Antiseptic, Johnson & Johnson Consumer Inc., Skillman, NJ, USA) have been studied extensively in clinical trials of six months or longer.7-16
Mechanical (medical) devices such as toothbrushes and dental floss and chemotherapeutic (drug) products such as toothpastes and mouthrinses have different functional characteristics and are considered under separate categories (manual interdental cleaners and chemotherapeutic products for control of gingivitis) within the American Dental Association’s (ADA) Council on Scientific Affairs Seal of Acceptance Program.17,18 In 2016, the ADA’s Council on Scientific Affairs modified the Seal of Acceptance program guidelines for both product categories. Under the revisions, both product categories have similar efficacy criteria requirements to fulfill for the Seal of Acceptance.18 Combined results for a product must demonstrate an average reduction in gingivitis of ≥10% (using the Modified Gingival Index (MGI)) or ≥ 15% (using the Löe and Silness gingival index) compared to the control group.19,20 Plaque measurements only require reductions that are statistically significantly different from the control group. The main difference between the categories is the required duration of clinical trials; interdental cleaning devices require two 30-day studies, whereas chemotherapeutic agents require two 3-month studies (prior to 2016, a six-month duration was required).18
When taking the chemotherapeutics guidelines into consideration and testing a combination of mechanical and chemotherapeutic agents, previous trials have had short durations (ie, two weeks evaluating EO mouthrinse vs flossing twice daily),21 intermediate durations (ie, eight weeks assessing cetylpyridinium chloride (CPC) and chlorhexidine (CHX) rinses vs flossing)22 and would not fulfill the more rigorous requirements (ie, longer duration) for chemotherapeutic agents. Bosma et al. report the comparative effectiveness of flossing or rinsing on plaque and gingivitis using a three-month timepoint in their examiner-blind, randomized, controlled clinical trial.23 Additionally, three studies of six-month duration conducted prior to the 2016 ADA guideline revisions demonstrated the benefits of EO mouthrinse and floss in combination.24-26 The lack of published studies of at least three months duration combining chemotherapeutic with mechanical interventions, confirms the need for studies that meet the longer term duration requirements (ie, the chemotherapeutic study requirement) according to current ADA guidelines.
The studies discussed above, with the exception of Bosma et al.,23 were unsupervised and did not monitor daily technique and product use. A search of the literature failed to identify supervised studies that were conducted for at least three months for both mouthrinse and floss. Within the context of a home-use study, including virtual supervision is a reasonable and sufficient method to help ensure use of product. In addition, to answer questions regarding the effectiveness of technique-sensitive practices such as dental flossing, virtual supervision also provides insights into study participants abilities and practices. The purpose of this 12-week clinical trial was to investigate the effects of various combinations of supervised mechanical and chemotherapeutic regimens on the prevention and reduction of plaque, gingivitis, and gingival bleeding.
Methods
This examiner-blind, randomized, parallel group, controlled clinical trial was conducted at Salus Research, Inc. (Fort Wayne, IN, USA), an American Dental Association (ADA) qualified site,27 from October 2020 to February 2021. The principles of the International Council on Harmonisation (ICH) Guidance for Good Clinical Practice (ICH E6 (R2)) were applied and the study protocol was approved by the Institutional Ethics Committee on research involving humans (IntegReview IRB, Austin, TX, USA). After receiving a thorough explanation of the study and the opportunity to ask questions in private, all participants provided written informed consent on a form which complied with the requirements of the Health Insurance Portability and Accountability Act. The study was registered on clinicaltrials.gov (registration number NCT04750005).
The randomization schedule was generated using a validated program created by the Biostatistics Department at Johnson & Johnson Consumer Inc. (JJCI, Skillman, NJ, USA). Participants were assigned in equal allocation to each treatment using a block randomization with block size of four; participants were assigned a unique randomization number that determined treatment assignment. The principal investigator (PI) and examiners were blinded to the treatments administered to participants. Personnel dispensing the test products or supervising their use did not participate in the examination of participants to minimize potential bias. During supervised use other staff members, including the PI /examiners, did not have access to the area where the product was being administered.
Sample
Participants were from the Fort Wayne, IN area and were selected for screening from the clinical site’s database on the basis of the trial’s inclusion and exclusion criteria, which included such items as gingivitis, bleeding, and periodontal involvement. Participants were males and females aged 18-60 years (age limited to 60 years by sponsor due to Covid-19 risk factors at the time of the study), in good general and oral health, without known allergies to commercial dental products, with at least 20 teeth with scorable facial and lingual surfaces. All participants had evidence of some gingivitis (although no minimum score on the MGI was required), were without evidence of advanced periodontitis, and had at least 10 percent bleeding sites based on the Expanded Bleeding Index (EBI) as determined by the screening/baseline examination.19,28 Participants were eligible for the study if they had no sites with >5 mm probing depth, and a maximum of three sites with 5 mm probing depths. Participants agreed to attend virtual smart-phone video daily sessions on weekdays for study procedures. Other inclusion criteria included the absence of fixed or removable orthodontic appliances or removable partial dentures; the absence of significant oral soft tissue pathology excluding plaque-induced gingivitis, (at the discretion of the PI). Female participants of childbearing potential had negative pregnancy tests (baseline and week 12) and agreed to use medically acceptable methods of birth control for one month prior to baseline and throughout the study. Participants were not permitted to have dental procedures unless needed as emergency treatment during the study.
The following conditions excluded participants from participation: having had a dental prophylaxis within four weeks prior to screening/baseline; needing antibiotics prior to dental treatment; use of certain medications within last month (antibiotics, anti-inflammatory or anticoagulant therapy within one month); use of chemotherapeutic oral care products within two weeks; being pregnant or lactating; use of smokeless tobacco, vaping or e-cigarettes or suspected substance abuse; and any other medical or psychiatric condition that would make the volunteer inappropriate for the study in the judgment of the PI.
Interventions
At baseline, all participants had a complete dental prophylaxis before being assigned to study products. Qualified participants were randomized into one of four treatment groups: 1) brush only (BO); 2) brush and rinse with fixed combination of four essential oils (4EO) (Listerine® Cool Mint® Antiseptic Mouthwash; JJCI, Skillman, NJ, USA) mouthrinse (BR); 3) brush and floss (BF); 4) brush, floss, and rinse with 4EO mouthrinse (BFR). Each participant received a soft-bristled manual toothbrush (ADA soft, flat-trim reference toothbrush, sourced through the ADA) and toothpaste (Colgate® Cavity Protection; Colgate-Palmolive, New York, NY, USA). Participants assigned to the flossing groups received an unflavored waxed dental floss (REACH® Waxed Unflavored Dental Floss; JJCI, Skillman, NJ, USA). Participants in rinsing groups received blinded bottles of 4EO mouthrinse and marked dosage cups. Instructions for use were provided at screening/baseline session. Participants assigned to a flossing group received specific instruction on flossing technique by a dental hygienist and had to demonstrate competency to them. All participants performed the first use of their regimen under supervision at the test site.
For the 12-week duration of the study, all participants performed their oral hygiene regimens at the beginning of each weekday under virtual supervision (smartphone) by study personnel. The second weekday use and the twice-daily usage on weekends/holidays were unsupervised. All participants were instructed to brush for one minute (timed) with a full ribbon of study dentifrice twice daily. The BR group brushed then rinsed with 20 mL of 4EO rinse for 30 seconds (timed) twice daily. The BF group brushed, then flossed as directed during the first daily oral hygiene session. In the evenings, these participants brushed but did not floss. The BFR group brushed, flossed as directed, then rinsed with 20 mL of 4EO rinse for 30 seconds (timed) during the first oral hygiene session. In the evenings, these participants brushed and rinsed but did not floss. Participants maintained diaries to document product use and brought all materials to the test site at weeks 4 and 12; diaries were checked, and floss and mouthrinse materials were weighed for compliance.
Assessments
Assessments were conducted at baseline, weeks 4 and 12. Prior to each visit, participants refrained from their product use for at least eight (but not more than 18) hours and did not eat for at least four hours before the visit. All assessment visits included review of the inclusion/exclusion criteria and concomitant medications, oral examination of hard and soft tissues, and adverse event monitoring before other measurements were taken. Each clinical assessment was performed consistently throughout the study by one trained and calibrated clinical examiner. Calibration of the examiner included an intra-examiner repeatability exercise performed yearly according to the site’s standard operating procedures for the specific assessment.
Clinical assessments were conducted in the following order: oral examination of hard and soft tissue for safety, MGI, six-site EBI, probing depth, bleeding on probing (BOP) (baseline and week 12 only), six-site Turesky modification of the Quigley-Hein Plaque Index (TPI), and Proximal Marginal Plaque Index (PMI).19,28-32 All plaque assessments for this trial were supragingival measures and probing depth and BOP were measured at six sites. Measurements were made at six-sites for each graded tooth (mesiofacial, facial, distofacial, mesiolingual, lingual, distolingual). Bleeding on probing measures were based on 1 = yes bleeding, 0 = no bleeding.
The primary efficacy endpoints were whole mouth mean MGI and TPI at week 12. Additional secondary efficacy endpoints at week 4 were whole mouth mean TPI, MGI, and EBI; marginal mean TPI, MGI and EBI at weeks 4 and 12; interproximal mean TPI, MGI, EBI at weeks 4 and 12; interproximal percent bleeding sites at weeks 4 and 12; interproximal mean PMI at weeks 4 and 12. Exploratory endpoints at week 12 were whole mouth and interproximal mean probing depth and bleeding on probing (BOP).
Statistical analyses
A sample size of 200 participants (50 per treatment group) was estimated to provide greater than 95% power to detect a population difference of 0.46 between BR and BF in mean MGI, assuming a population standard deviation of 0.44; and greater than 95% power to detect a population difference of 0.54 between BR and BF in mean TPI, assuming a population standard deviation (SD) of 0.37. The population within-treatment SD and differences between population means were based on results from studies using the same examiners as the current study.23 This sample size also provides 95% power for detecting a standardized effect size (difference between treatment means divided by SD) of 0.78 (MGI) for BFR versus BF and greater than 99% power to detect a standardized effect size of 1.5 (TPI) for BFR versus BF. These standardized effect size estimates were based on the study sponsor’s historical database for MGI and TPI clinical trial data. Sample sizes were estimated using PASS version 14.0.4 (NCSS, LLC, Kaysville, UT, USA).
Treatments were compared using a mixed effects model for repeated measures (MMRM) approach, considering within-participant correlation as unstructured and with model terms for baseline as a covariate, treatment, visit, treatment by visit interaction, and baseline by visit interaction.33,34 For key comparisons of BR versus BF and BFR versus BF at 12 weeks, the familywise type I error rate was strongly controlled at one-sided 2.5% by separately applying a fixed sequence approach for BR versus BF and BFR versus BF comparisons, and testing at the one-sided 1.25% significance level at each step within those sequences. For BR versus BF, non-inferiority with respect to MGI and TPI was assessed first. Provided that non-inferiority was demonstrated with respect to both MGI and TPI, superiority of BR versus BF was tested with respect to TPI and then MGI, and subsequently non-inferiority of BR versus BF was tested with respect to EBI. If the null hypothesis was not rejected at any step in the sequence, any further testing was considered exploratory. For BFR versus BF, superiority was similarly tested with respect to TPI, followed by MGI, and then by EBI. All comparisons outside the fixed-sequence procedure were tested at the 2.5% significance level, one-sided.
Non-inferiority for BR versus BF, within the fixed sequence referenced above, was assessed by testing the null hypothesis H0 ((μBR- μB) ≥ (1/2) (μBF- μB)) versus alternative (one-sided) hypothesis H1 ((μBR- μB) ≥ (1/2) (μBF- μB)). Rejection of H0 in favor of H1 demonstrates statistically that BR maintains a majority of the effect of BF, where the effect of BR is μBR-μB, and the effect of BF is μF- μB. The ratio (μBR- μB)/(μBF-μB) was further explored using Fieller confidence intervals if μBF- μB was significantly different from 0. (Fieller intervals are not presented in this paper, as superiority testing revealed superiority for BR versus BF, and therefore further exploration of the ratio (μBR- μB) / (μBF- μB) was not necessary.)
Demographic and baseline characteristics were compared across treatment groups using analysis of variance (ANOVA), Chi-square test, or Fisher’s exact test. SAS version 9.4 software (SAS Institute, Cary, NC, USA) was used for statistical analyses.
Results
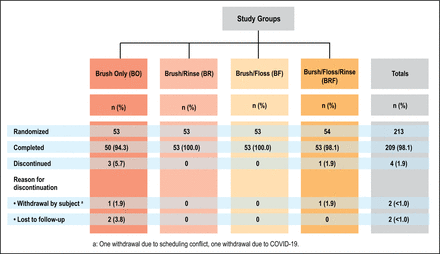
Of the 213 randomized participants, 209 completed the study. Participants were randomized into four treatment groups: BO (n=53), BR (n=53), BF (n=53), and BFR (n=54). Two participants withdrew and two were lost to follow up. The sample distribution is shown in Figure 1. Demographic and baseline characteristics are presented in Table I. There were no statistically significant differences in baseline measurements among the groups, with the exception of mean whole mouth TPI. Variation in baseline index scores was accounted for by using the prespecified covariate adjustment in the statistical model.
Participant distribution (n= 213)
Participant demographics and baseline characteristics (n=213)
Efficacy: Primary endpoints
Whole Mouth Mean TPI and MGI at Week 12
At week 12, the BR and BFR groups demonstrated significantly reduced whole mouth mean TPI compared to the BO group (35.8% reduction and 32.8% reduction, respectively, p<0.001). The whole mouth mean TPI in the BF group was not significantly different from the BO group (p=0.935). In addition, compared to the BF group, the BR and BFR groups demonstrated significantly reduced whole mouth mean TPI (38.3% and 35.5%, p<0.001). The whole mouth mean TPI was not significantly different between the BR and BFR groups (p=0.861) (Table II).
Whole Mouth Mean TPI after 4 and 12 Weeks: Full analysis set
As compared to the BO group, all three groups had significantly reduced whole mouth mean MGI at week 12; BR group reduced by 50.8%, (p<0.001); BF group by 9.2% (p=0.013); BFR group by 54.1% (p<0.001). In addition, compared to the BF group, the BR and BFR groups demonstrated significantly reduced whole mouth mean MGI by 45.8% and 49.5%, respectively (p<0.001). The whole mouth mean MGI was not significantly different between the BR and BFR groups (p=0.203) (Table III). As described in the methods, the whole mouth mean TPI and whole mouth mean MGI non-inferiority and superiority comparisons between BR and BF were performed sequentially, first non-inferiority then superiority. Because both non-inferiority and superiority were demonstrated, only superiority is discussed to avoid redundancy.
Whole Mouth Mean MGI after 4 and 12 weeks: Full analysis set
Efficacy: Secondary endpoints
Whole Mouth Mean EBI and Percent Bleeding Sites at Week 12
Compared to the BO group, all three groups demonstrated significantly reduced whole mouth mean EBI at week 12: BR group by 71.0% (p<0.001); BF group by 17.5% (p=0.003); BFR group by 78.2% (p<0.001). The BR was demonstrated as non-inferior to the BF (p<0.001). In addition, compared to the BF group, the BR and BFR groups significantly reduced whole mouth mean EBI (64.9% and 73.6%, respectively, p<0.001). The whole mouth mean EBI did not differ significantly between the BR and BFR groups (p=0.127).
Regarding gingival bleeding, when compared to the BO group, all three groups demonstrated significantly reduced whole mouth percent bleeding sites at week 12; BR group by 68.9% (p<0.001); BF group by 14.0% (p=0.006); BFR group by 75.5% (p<0.001). In addition, as compared to the BF group, the BR and BFR groups demonstrated significantly reduced whole mouth percent bleeding sites (63.8% and 71.5%, respectively, p<0.001). Whole mouth percent bleeding sites did not differ significantly between the BR and BFR groups (p=0.112).
While it appears redundant to present non-inferiority comparisons as well as superiority comparisons of BR with BF for whole mouth mean EBI, both results are presented because this non-inferiority comparison (but not the corresponding superiority comparisons) was one of the set of key comparisons controlled strongly at the one-sided 2.5% familywise error rate.
Interproximal TPI, MGI, EBI, Percent Bleeding Sites at Week 12
The BR and BFR groups demonstrated significantly reduced interproximal mean TPI as compared to the BO group (26.9% reduction and 24.9% reduction, respectively, p<0.001). The BF group did not differ significantly from the BO group (p=0.976). In addition, compared to the BF group, the BR and BFR groups showed significantly reduced interproximal mean TPI (30.3% reduction and 28.4% reduction, respectively, p<0.001). The interproximal mean TPI did not significantly differ between the BR and BFR groups (p=0.793) (Table II).
All three groups demonstrated significantly reduced interproximal mean MGI as compared to the BO group at week 12: BR group by 42.7% (p<0.001); BF group by 8.9% (p=0.006); BFR group by 44.0% (p<0.001). In addition, compared to the BF group, the BR and BFR groups demonstrated significantly reduced interproximal mean MGI (37.1% reduction and 38.5% reduction, respectively, p<0.001). The interproximal mean MGI did not differ significantly between the BR and BFR groups (p=0.357) (Table III).
As compared to the BO group, all three groups demonstrated significantly reduced interproximal mean EBI at week 12: BR group by 71.5% (p<0.001); BF group by 19.2% (p=0.002); BFR group by 81.4% (p<0.001). In addition, the BR and BFR groups significantly reduced interproximal mean EBI compared to the BF group (64.7% reduction and 77.0% reduction, respectively, p<0.001). The interproximal mean EBI did not differ significantly between the BR and BFR groups (p=0.068) (Table IV).
Secondary efficacy endpoints
Compared to the BO group, all three groups demonstrated significantly reduced interproximal percent bleeding sites at week 12: BR group by 68.7% (p<0.001); BF group by 16.1% (p=0.004); BFR group by 79.3% (p<0.001). The BR group demonstrated significantly reduced interproximal percent bleeding sites as compared to the BF group (62.8% reduction, p<0.001). In addition, the BFR group demonstrated significantly reduced interproximal percent bleeding sites as compared to the BF group (75.4% reduction, p<0.001), but not compared to the BR group (33.8% reduction, p=0.04) (Table IV).
Interproximal Mean PMI at Week 12
The BR and BFR groups demonstrated significantly reduced interproximal mean PMI as com-pared to the BO group (29.6% reduction and 24.9% reduction, respectively, p<0.001). The BF group did not differ significantly from the BO group (p=0.894). In addition, as compared to the BF group, the BR and BFR groups demonstrated significantly reduced interproximal mean PMI (31.9% reduction and 27.5% reduction, respectively, p<0.001). The interproximal PMI did not differ significantly between the BR and BFR groups (p=0.953) (Table III).
All other secondary endpoints measured at weeks 4 and 12 are presented in Tables II-IV. The exploratory endpoints of whole mouth and interproximal mean probing depth and mean BOP at week 12 are presented in Table V. All statistical tests comparing the BR and BFR groups to the BF group in this study, apart from the non-inferiority tests for BR and BF, were one-sided assessing the benefit of BR or BFR to BF. Using the one-sided approach, interproximal mean BOP for the BR group as compared to the BF group did not show statistically significant reductions in favor of BR (p=0.976) after 12 weeks. To more completely evaluate the relative benefits of flossing given the magnitude and direction of the observed difference, a two-sided test was applied for this comparison. Based on this statistical test, the BF group had a significantly lower mean interproximal BOP than the BR group (p=0.049) after 12 weeks.
Exploratory endpoints: Whole mouth and interproximal probing depth and bleeding on probing at week 12.
Safety
Of the treatment-emergent adverse events (TEAE) the PI classified as “probable” or “very likely” caused by the study product, one participant experienced moderate lip mucosa desquamation, and two participants in the BR group experienced mild oral mucosal desquamation. Four participants in the BFR group experienced mild oral mucosal desquamation. All were single episodes that required no treatment and resolved. All other TEAEs (angular cheilitis, coated tongue, ulcer, and mouth ulceration due to food burn) were also single events, either mild or moderate in severity, that resolved without treatment. No participants discontinued participation in the study due to adverse events. No deaths and no serious AEs were reported.
Discussion
The purpose of this long-term (12-week) clinical trial was to investigate the effects of various combinations of supervised mechanical and chemotherapeutic regimens on the prevention and reduction of plaque, gingivitis, and gingival bleeding. Participants using a mouthrinse containing a fixed combination of four essential oils, in combination with toothbrushing or with toothbrushing and flossing, demonstrated statistically significant reductions in supragingival plaque, gingivitis, and gingival bleeding as compared to toothbrushing only and compared to toothbrushing and flossing at the end of 12 weeks. Furthermore, using dental floss in addition to toothbrushing (BF) provided no measurable plaque reduction as compared to toothbrushing alone (BO) but did provide reductions in gingivitis and gingival bleeding when compared to BO at 12 weeks. Although the Turesky modification of the Quigley-Hein Plaque Index (TPI) is a more widely utilized supragingival plaque index in clinical trials, use of the Proximal Marginal Plaque Index (PMI) in this study produced a similar pattern in plaque reduction in comparison to TPI, helping to confirm the robustness of the findings.
While the use of dental floss was not shown to reduce supragingival plaque in this study, it was shown to statistically significantly reduce several whole mouth and interproximal measures (i.e. mean MGI, mean EBI, marginal gingival bleeding, and percent of bleeding sites based on EBI) compared to brushing alone, but not as effectively as the EO mouthrinsing regimens. This finding suggests that while the mechanical action of flossing may affect the plaque mass for a short period of time that was long enough to have an impact on gingival health, its effect was not long enough to measure significant plaque reduction at 8-18 hours. These results are consistent with findings of a 12-week clinical trial conducted by Bosma et al.23
Although it was an exploratory outcome measure in this supervised clinical trial, the BOP results had clinical implications. Participants assigned to flossing as part of their oral care regimen (BFR and BF) had statistically significant reductions in whole mouth and interproximal mean BOP at 12 weeks compared to toothbrushing only (BO). Compared to BR at 12 weeks, the BFR and BF groups demonstrated statistically significantly reduced interproximal mean BOP and only the BFR group demonstrated statistically significantly reduced whole mouth mean BOP. These findings were consistent with the supervised flossing groups in a study by Bosma et al.23 A potential explanation for this could be the deeper interproximal subgingival access and the mechanical plaque disruption that flossing may provide compared to rinsing with a 4EO mouthrinse.
Periodontal diseases are a result of complex interactions of multiple factors. Evaluating an individual’s periodontal health should take into consideration more than plaque and bacterial control.35 In a review of the histological and clinical determinants of periodontal health, Lang and Bartold provide a definition for both the intact and reduced periodontium and state that BOP is the best parameter for monitoring health or inflammation of the gingival tissue.35 Although the severity of the gingival bleeding and the amount of plaque accumulation are associated with one another, it has been suggested that BOP may be an earlier sign of gingivitis than erythema and edema.35 In the presence of BOP, it is impossible to have pristine periodontal health.35
To determine the best adjunctive routine in addition to brushing only, multiple disease measures (MGI, bleeding, probing depth, BOP) were considered at the 12-week timepoint of this study. When comparing BR versus BF for these endpoints at week 12, BR was statistically significantly better than BF for reducing interproximal MGI and percent bleeding sites but BF was significantly better than BR for reducing interproximal BOP. When comparing BFR to BR, BFR was statistically significantly better than BR for reducing interproximal BOP but not for other interproximal measures. Moreover, BFR provided statistically significant reductions in interproximal MGI, percent bleeding sites, and probing depth compared to BF. Whole mouth results followed a similar pattern.
Considering the evidence generated in this study and evidence from an earlier study,26 twice daily brushing, daily flossing, and twice daily rinsing with an essential oil mouthrinse should be considered when advising patients on the management of plaque, gingivitis and gingival bleeding. The current clinical study provides additional data-driven evidence to assist healthcare providers in recommending plaque and gingivitis control methods as part of their patients’ oral care regimens.
Recognizing that effective flossing can be a difficult task that requires functional bilateral dexterity and skill, a component of the current study explored the relationship between manual dexterity and clinical outcomes.36 Another component of the current study surveyed participants regarding their oral hygiene habits at baseline.37 Results of all components of this study provide dental professionals with information and insights to better counsel patients about daily oral hygiene regimens.
Limitations and future research
The study was conducted from October 2020 to February 2021, during the COVID-19 pandemic and this may have influenced the volunteers and their mindset (e.g. age, risk tolerance) for participation in this study. Fear, risk aversion, or other concerns, perhaps related to medical status, during this time may have discouraged certain types of individuals from participating in the study. The sample was limited to individuals who volunteered to be part of a clinical study and may not be representative of the general population. The clinical site specifically recruited participants with evidence of some gingivitis and without evidence of severe periodontitis. Thus, the results would not be generalizable to individuals who are on either end of the periodontal health/disease spectrum.
Differences between supervised two (brushing and rinsing) versus three (brushing, flossing, rinsing) step oral care routines, once versus multiple daily occasions of flossing, and dental floss versus other interdental aids were not explored. Evidence is lacking for the long-term clinical benefit of flossing multiple times a day. Additionally, there are multiple aids for interdental cleaning which include interdental brushes. A 2014 workshop of internationally recognized dental experts sponsored by the European Federation of Periodontology concluded that flossing should only be recommended for sites where interdental brushes would not be able to pass through the interproximal areas without causing trauma, eg, sites where attachment loss is not present.38 Considering the positive effect on BOP by flossing shown in this study, future research exploring the benefits of flossing on this measure is indicated. Future studies should also investigate the combination of interproximal brushes and mouthrinses as related to gingival health and the parameters investigated in this trial.
Conclusions
Virtually supervised oral care regimens that included a mouthrinse containing a combination of four essential oils (BR and BFR), significantly reduced plaque, gingivitis, and gingival bleeding as compared to toothbrushing only (BO) and brushing and flossing (BF) after 12 weeks. Gingivitis and gingival bleeding were significantly reduced in the BF group; however, plaque levels were not reduced after 12 weeks. The BF regimen was not significantly different from BO after 12 weeks. These data provide evidence for dental healthcare professionals to recommend a three-part oral hygiene regimen of brushing, flossing and mouthrinsing to their patients.
Acknowledgments
Medical writing support was provided by Carol Feinberg Consulting LLC and was funded by JJCI. The authors would like to acknowledge Michael Lynch, DMD, PhD and Victoria Aleles of JJCI for critically reviewing the manuscript and Kathleen E. Boyle of 4 Learning Group, LLC for managing final reviews and submission-related activities.
Footnotes
This manuscript supports the NDHRA priority area, Client level: oral health care (new therapies and prevention modalities).
Disclosures
Johnson & Johnson Consumer Inc. (JJCI; Skillman, NJ, USA) sponsored this clinical trial and was responsible for the study design and the collection, analysis, and interpretation of the data. Mary Lynn Bosma, James A. McGuire, Kathleen McAdoo, and Alicia DelSasso are employees of JJCI. Jeffery Milleman and Kimberly Milleman are principals at Salus Research, Inc., Fort Wayne, IN, USA and received grants from JJCI and conducted the study on behalf of JJCI. Kaylie Wills is an employee of Salus Research, Inc.
- Received February 28, 2022.
- Accepted May 10, 2022.
- Copyright © 2022 The American Dental Hygienists’ Association
This article is open access and may not be copied, distributed, or modified without written permission from the American Dental Hygienists’ Association.