Abstract
Purpose Individuals with cystic fibrosis (CF) present with multiple condition-specific risk factors for periodontitis including CF-related diabetes, chronic inhaled treatments that induce xerostomia, and increased systemic inflammation because of frequent lung infections. General factors like age, oral hygiene, and diet may also contribute to the risk of periodontitis. However the relative importance of these specific risk factors and periodontitis in individuals with CF has not yet been evaluated. The purpose of this pilot study was to assess the associations between CF condition-specific and general risk factors and the prevalence of periodontitis in adults with CF.
Methods This cross-sectional pilot study was designed to assess a multifactorial model of periodontitis risk factors in a population in adults with CF who were recruited from the University of Washington Adult CF center. Periodontitis was defined using the Centers for Disease Control and Prevention and the American Academy of Periodontology (CDC/AAP) case definition. Risk factors included condition-specific and general factors. Differences between participants with moderate/severe periodontitis and those with no/mild periodontitis was assessed using the Mann-Whitney test, the Fisher’s exact test, and the exact chi-square test (α=0.05).
Results Thirty-two participants were enrolled. Twenty-eight percent of the participants had moderate periodontitis, 72% had no/mild periodontitis; none of the participants had severe periodontitis. There were no significant differences in condition-specific factors between between the two study groups. Participants with moderate periodontitis were older (p=0.028) and reported daily flossing in higher proportions than those with no/mild periodontitis (p=0.023).
Conclusions The findings from this pilot study suggest that future research is needed to determine whether sociodemographic and other general risk factors are more important contributors to periodontitis risk than CF-specific factors.
INTRODUCTION
Cystic fibrosis (CF) is a life-limiting autosomal recessive disease that affects multiple organ systems1. The main cause of morbidity and mortality in individuals with CF is respiratory disease resulting from accumulation of thick mucus in the lungs.2 Cystic fibrosis impacts individuals of every racial group but is most prevalent in Caucasians with an estimated incidence of one in every 3,500 live births. In the United States (US) there are more than 35,000 individuals with CF and about 1,000 new cases diagnosed every year.3 Historically, individuals with CF died during childhood. However, currently because of advancements in diagnosis and treatment, more than half of the CF population is 18 years and older, and the median predicted survival of individuals with CF is 50 years.1
Periodontitis is a result of host immune response to dysbiosis of the commensal oral microbiota leading to inflammation and disease of the periodontium (periodontal fibers and alveolar bone.4 If left untreated this disease process causes deep periodontal pockets and leads to alveolar bone loss and subsequent loosening and the eventual loss of teeth. Periodontitis is the leading cause of tooth loss in US adults ages 30 years or older5 and is independently associated with increased systemic inflammation that can compromise general health.6 As such, periodontitis is a potential source of bacteria that could negatively impact the respiratory health of individuals with CF. Numerous research studies have demonstrated an increased risk of micro-aspiration of oral bacteria and links between oral infections and decreased lung function in individuals with lung diseases.7-11 However, the potential links between oral health and respiratory health of adults with CF have not yet been reported in the literature.
Health conditions specific to CF such as CF-related diabetes, increased systemic inflammation due to frequent lung infections, inhaled treatments that can induce xerostomia, bone disease, anxiety, and depression potentially increase periodontitis risk in this population.4,12-14 General factors such as age, oral hygiene, and diet may also contribute to periodontitis risk.15,16 Despite this risk profile, most studies report lower periodontal disease prevalence in individuals with CF as compared to non-CF control groups and attribute this difference to the frequent use of antibiotics to treat chronic lung infections.17-19 However, the association between antibiotic use and periodontal disease has not yet been formally tested in individuals with CF. Collectively, these studies form the current knowledge base of CF and risk of periodontal disease. These studies however are outdated (1977-2009), do not include a comprehensive periodontal examination, and are primarily focused on children and adolescents, an age group for which the prevalence of periodontitis is low.17-19 Only more recent study has focused on adults with CF and reported more than one-third of the study population had periodontitis.20 To date, no study has evaluated whether the CF condition-specific and general factors described above are associated with periodontitis in individuals with CF.
Understanding the oral disease prevalence and oral health needs of individuals with CF is evolving. There is a critical need to further develop and refine condition-specific standards of dental clinical care for this medically vulnerable population. The purpose of this pilot study was to assess the associations between CF condition-specific and general factors and the prevalence of periodontitis in adults with CF.
METHODS
This cross-sectional pilot study consisted of a convenience sample of adults with CF (age ≥ 18 years) recruited from a single adult CF center in Washington State from November 2019 to June 2021. The study included individuals who were able to provide informed consent and complete the study in English. There were four exclusion criteria. First, because of the high contagion risk for adults with CF, the study excluded adults with active infection with Burkholderia cenocepacia or Mycobacterium abscessus in the prior two years. Second, to reduce potential bias of adverse impact of pulmonary exacerbations and steroids on blood glucose level (a predictor variable of interest), the study excluded adults with pulmonary exacerbation requiring systemic steroids in the prior 4 weeks. Third, because smoking is a well-documented risk factor for periodontitis, and smoking is rare among adults with CF, the study excluded adults with a history of smoking. Fourth, the study excluded adults who required prophylactic antibiotics before dental procedures to avoid the risk of subacute bacterial endocarditis. This study was approved by the University of Washington Institutional Review Board (IRB); protocol number: 00007976.
A HIPAA waiver of consent was approved by the IRB to allow for a medical record screening to identify eligible participants. Eligible adults with CF were invited to participate in the study by phone or email. A single two-hour study visit was then scheduled at the dental research clinic at the University of Washington. The study followed the Cystic Fibrosis Foundation and the American Dental Association guidelines for infection control. Informed consent and HIPAA authorization were obtained from participants to allow for subsequent abstraction of medical record data. For participants with unmet dental treatment needs, a treatment referral was provided. At the end of the study visit, study participants were provided oral hygiene instruction, an oral hygiene kit, and $95 as a reimbursement for transportation and compensation for the participant’s time.
PROCEDURES
Five study procedures were designed to collect periodontal assessments and additional data on CF condition-specific and general risk factors. Study procedures were performed by two researchers and included an oral health survey, diet assessment, oral hygiene assessment, periodontal screening, and medical record abstraction. The 17-item oral health survey included questions focused on sociodemographic factors (race, ethnicity, household income, education level, food insecurity, dental insurance), history of dental care use, and oral hygiene practices. The survey was designed using REDCap™,21 and was tested on a different CF population prior to use for this study. Participants used a designated iPad to complete the survey.
Diet assessments
A 24-hour dietary recall was collected for each participant using the Automated Self-Administered 24-Hour (ASA-24) dietary assessment tool. The ASA-24 is a web-based tool developed by the National Cancer Institute and is a practical and cost-effective alternative to the standard interviewer-administered 24-hour dietary recall.22 Study participants completed the ASA-24 based on their dietary intake on the day prior to the study visit. The current study specifically focused on the dietary inflammatory potential using the Dietary Inflammatory Index (DII). A description of the design and development of the original DII has been previously reported.23 The DII computes the inflammatory potential of food consumed based on the impact of certain food and nutrients on six established inflammatory biomarkers (IL-1ß, IL-4, IL-6, IL-10, TNF-α, and C-reactive protein). The overall DII score is a continuous measure drawn from a possible total of 45 food parameters. Positive DII scores indicate a pro-inflammatory diet, and negative scores imply an anti-inflammatory diet. To calculate DII score for study participants, the following food parameters were evaluated: total calories, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, omega-3 fatty acids, omega-6 fatty acids, protein, carbohydrate, fiber, alcohol, cholesterol, niacin, thiamin, vitamin A, vitamin B2, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, iron, magnesium, selenium, zinc, folic acid, ß carotene, and caffeine.23
Oral hygiene Assessments
Oral hygiene was assessed using the Silness-Löe Plaque Index.24 The presence of dental plaque was assessed on four surfaces (mesial, distal, buccal, and lingual) of six teeth (maxillary right first molar, maxillary right lateral incisor, maxillary left first premolar, mandibular left first molar, mandibular left lateral incisor, and mandibular right first premolar). A tooth surface was scored in the following manner: 0=no plaque;1=plaque presence may only be recognized by running a probe across the tooth surface above the gingival margin; 2=moderate accumulation of soft deposits visible with the naked eye; 3=an abundance of soft matter within the gingival pocket, on the tooth, or gingival margin. Consistent with previous studies, the mean plaque score was computed across examined surfaces and used to determine the oral hygiene category: 0.00-0.09 =excellent oral hygiene; 0.10-0.99=good oral hygiene; 1.00-1.99=fair oral hygiene; 2.00-3.00=poor oral hygiene.24
Periodontal screening
Each participant received a full-mouth periodontal screening conducted by a trained and calibrated registered dental hygienist.25 Prior to the periodontal probing, the dental examiner confirmed there were no contradictions for the procedure. Periodontal screenings were completed using a periodontal probe (UNC-15). Two periodontal measures were assessed at six sites per tooth (distobuccal, mid-buccal, mesiobuccal, distolingual, mid-lingual, and mesiolingual) for all teeth present, excluding third molars.26 The first measure was periodontal pocket depth (PD), defined as the distance in millimeters (mm) from the free gingival margin to the periodontal sulcus base. Second was the gingival margin level, defined as the distance in mm from the free gingival margin to the cementoenamel junction. Gingival margin level takes a negative value if the free gingival margin is apical to the cementoenamel junction (i.e., on the tooth root); a value of zero if the free gingival margin is at the level of cementoenamel junction; and a positive value if the free gingival margin is coronal to the cementoenamel junction (i.e., on the tooth crown). All measurements of PD and gingival margin level were rounded to the lowest whole mm. Finally, clinical attachment loss (CAL) was calculated by subtracting the gingival margin level from PD using the following equation: CAL=PD – (+/−) gingival margin level.
Medical record abstraction
After the in-person visit, the following information was abstracted from the electronic medical record to define the study variables: sociodemographic data (e.g., age, sex, and health insurance type); medical data including CF-transmembrane conductance regulator (CFTR) genotype, most recent forced expiratory volume in one second percent predicted (FEV1% predicted), comorbidities (CF-related diabetes, osteoporosis and/or osteopenia, anxiety, depression), glycated hemoglobin (HbA1c), body mass index (BMI), C-reactive protein, and current medication use (CFTR modulator, antibiotic, inhaled medications). Cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapy was defined as: triple CFTR modulator therapy (elexacaftor/tezacaftor/ivacaftor), other CFTR modulator therapy (ivacaftor, lumacaftor/ivacaftor, or tezacaftor/ivacaftor), or none. Antibiotic use was defined as the current use of oral or inhaled antibiotic (yes/no).
Outcome measure
Periodontitis was defined using the Centers for Disease Control and Prevention and the American Academy of Periodontology (CDC/AAP) case definition, which is based on PD and CAL.27 The (CDC/AAP) case definition of mild periodontitis is the presence of two or more interproximal sites with CAL ≥3 mm and two or more interproximal sites with PD ≥4 mm (not on the same tooth), or one site with PD ≥5 mm. Moderate periodontitis is defined as the presence of two or more interproximal sites with CAL ≥4 mm (not on the same tooth), or two or more interproximal sites with PD ≥5 mm (not on the same tooth). Severe periodontitis is defined as the presence of two or more interproximal sites with CAL ≥6 mm (not on the same tooth) and ≥1 interproximal site with PD ≥5 mm. Participants were grouped as having moderate/severe periodontitis versus no/mild periodontitis. This grouping is commonly used for studies examining the association between periodontitis and chronic medical conditions.28,29
Predictor variables
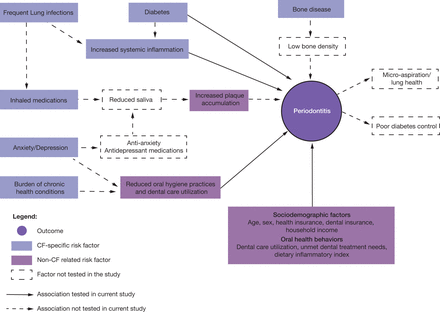
The multifactorial oral disease risk factor model for individuals with CF developed by Chi et al.11 was adapted to identify risk factors for periodontitis in adults with CF (Figure 1). This study focused on CF-specific factors including CFTR genotype, CF-related diabetes, bone disease, anxiety, depression, antibiotic use, inhaled medication use, FEV1% predicted, BMI, and C-reactive protein. Other general risk factors for periodontitis were also evaluated and were grouped in two domains: sociodemographic factors and oral health behaviors.
Conceptualized multifactorial risk factor model for periodontitis in adults with CF
Data analysis
Descriptive statistics were used to report on study variables using means and standard deviations (SD) for normally distributed quantitative variables, medians and interquartile ranges (IQR) for non-normally distributed quantitative variables, and frequencies and percentages for categorical variables.
For hypothesis testing, the study first assessed differences in condition-specific factors between participants with moderate/severe periodontitis and those with no/mild periodontitis. The independent samples t-test was used for normally distributed quantitative variables, the Mann-Whitney test was used for non-normally distributed quantitative variables, the Fisher’s exact test was used for binary variables, and the exact chi-square test was used for categorical variables. Second, differences in general risk factors between the two study groups (moderate/severe vs no/mild periodontitis) using the same bivariate tests for condition-specific factors described previously. Third, the study assessed the associations between potential risk factors (condition-specific factors, general factors) and clinical measures of periodontitis (PD, CAL). The Spearman rank correlation was used for continuous variables, the Mann-Whitney test was used for binary variables, and the Kruskal-Wallis test was used for categorical variables. As this was a pilot study and hypothesis generating, no adjustments were made for multiple statistical tests. Statistical significance was set at α=0.05 and all tests were two-sided.
RESULTS
Study population
Thirty-two participants were enrolled. The median (IQR) age at the time of the study visit was 30 (28 – 38) years (range: 22 to 63 years); 63% were female, 97% were White, and 6% were Hispanic or Latino. In terms of insurance coverage, 25% were publicly insured (Medicaid or Medicare), 75% had private or dual health insurance (both public and private), and 91% had dental insurance. Nine percent reported food insecurity, 28% had an annual household income less than $70,000, and 66% had completed at least a 4-year college degree. Sample demographics are shown in Table I.
Demographics (n=32)
Forty-one percent of participants were homozygous for the F508del CFTR mutation, 47% were F508del heterozygous, and 13% had other mutations. The range of FEV1% predicted was 39% – 114%. Forty-seven percent of participants had CF-related diabetes, 31% had bone disease, 28% had anxiety, and 31% had depression (Table II). The median (IQR) of BMI was 23.1 (21.1 – 27.4) kg/m2; the median (IQR) of HbA1c was 5.6 (5.4 – 6.2) %; and the median (IQR) of C-reactive protein was 2.1 (0.7 – 5.7) mg/L.
Medical, and dental characteristics (n=32)
In terms of oral hygiene status, 22% of study participants had excellent oral hygiene, 72% had good oral hygiene, 6% had fair oral hygiene, and none were found to have poor oral hygiene. Twenty-five percent had not been to a dentist in over a year, and another 25% could not get needed dental treatment because of lack of insurance or inability to pay for dental care. Eighty-eight percent reported tooth brushing at least once per day, and 25% reported daily flossing (Table II).
Periodontitis prevalence and risk factors
Twenty-eight percent of study participants had moderate periodontitis; none were found to have severe periodontitis (Table II). The remaining 72% had no/mild periodontitis. There were no differences in condition-specific factors between participants with moderate periodontitis and participants with no/mild periodontitis (Table III). In terms of general factors, participants with moderate periodontitis were significantly older than those with no/mild periodontitis (median age: moderate periodontitis 39 years vs no/mild periodontitis 30 years; p=0.026) (Table IV). The group with moderate periodontitis had a higher proportion of participants who reported daily flossing compared to the group with no/mild periodontitis (moderate periodontitis 55.6% vs no/mild periodontitis 13%; p=0.023).
Condition-specific factors and periodontitis (n=32)
Sociodemographic factors and oral health behaviors and periodontitis (n=32)
Regarding clinical measures of periodontitis, age and daily flossing were associated with increased CAL (age, r=0.39, 95% CI=0.034,0.67; p=0.028), (median CAL: daily flossing 2.0mm vs no daily flossing 1.5mm; p=0.004) (Table V). No other factors, CF-specific or general, were associated with clinical measures of periodontitis.
Associations between condition-specific and general factors and clinical measures of periodontitis (n=32)
DISCUSSION
This pilot study aimed to assess the associations between CF condition-specific and general factors and the prevalence of periodontitis in adults with CF. The findings showed that CF condition-specific factors were not associated with periodontitis. However, general factors such as age and daily flossing habits were associated with increased periodontitis. These preliminary findings suggest that sociodemographic factors and oral health behaviors may be more important contributors to periodontitis risk in adults with CF than condition-specific factors.
No CF condition-specific factors were significantly associated with periodontitis. Factors such as diabetes, increased systemic inflammation, and frequent use of inhaled treatments have been shown to contribute to periodontitis risk4,12-14 but were not associated with periodontitis for adults with CF in this pilot study. These findings were inconsistent with non-CF studies that have shown these factors to be associated with increased risk of periodontitis through a variety of mechanisms (Figure 1). These include systemic mechanisms like the hyperglycemia-induced inflammation present in diabetes,14 local mechanisms such as reduced saliva and increased dental plaque accumulation associated with dry mouth-inducing medications,12,30 and behavioral mechanisms like reduced oral hygiene practices and lack of dental care utilization associated with chronic medical conditions.13 The most likely explanation is that the study participants had attributes that offered protection against periodontitis, including high income, near normal median lung function, dental insurance, frequent preventive dental care, and good oral hygiene habits. In addition, participants with diabetes had near normal hemoglobin A1c levels, which would also protect against periodontitis.5,15,31,32 Furthermore, the current study findings showed no association between antibiotic use and periodontitis. This is an important finding in light of the numerous studies implicating antibiotics as the reason individuals with CF are less likely to have periodontal disease.17-19 Future studies should increase the diversity of participants by including adults with advanced lung disease and adults with lower socioeconomic status to verify whether these pilot study findings are generalizable to all adults with CF.
The study identified two risk factors associated with periodontitis in participants with CF that are similar to findings in the general population. First, older participants with CF were more likely to have moderate periodontitis. Age was also positively associated with CAL (i.e., more attachment loss with older age). Age is a well-defined periodontitis risk factor in the general population.5 The association between age and periodontitis is not necessarily causal (i.e., periodontitis is not a natural outcome of aging). Rather age represents the cumulative effect of untreated inflammation and continued exposure to risk factors like bone disease, elevated glucose levels, and systemic inflammation associated with chronic comorbidities that are more prevalent in older age.33 The second risk factor that was significantly associated with periodontitis was daily flossing. Participants with moderate periodontitis reported more daily flossing compared to those with no/mild periodontitis. Daily flossing was also associated with increased CAL. A potential explanation for this paradoxical finding is that participants with moderate periodontitis may have been aware of their condition and as a result were more committed to interdental cleaning to prevent further disease. The latest European Federation of Periodontology statement concluded that flossing is only efficient in areas with no CAL, and that flossing should only be recommended for areas where an interdental brush will not pass through the interproximal area without trauma.6 However, the cross-sectional design of this study precludes the full interpretation of this finding. Longitudinal studies are needed to evaluate whether flossing and other interdental aids prevent further CAL and progression of severe periodontitis in adults with CF.
It is important to ensure optimal oral health for all adults with CF due to the potential risk of micro-aspiration of oral bacteria and because periodontitis can act as a systemic inflammatory stressor which could further compromise general health.34 Efforts to promote oral health may be particularly critical for older adults with CF as the current findings show increased risk of periodontitis with age. Older age is also associated with other oral diseases like dental caries, candidiasis, and oral cancers.35-37 Ensuring optimal oral health for adults with CF is particularly important in the context of increased survival as a result of highly effective CF therapeutics.3 Promotion of oral health for adults with CF can be enhanced by incorporating oral disease screening and oral health education as part of routine CF medical care. The CF medical care team could be trained to conduct oral screenings, or the CF care team could include a dental hygienist. This would be especially beneficial for those with limited dental care access, for whom routine CF clinical visits might be their only available professional health care.
There were four main study limitations. First, the study had limited power due to the small sample size and lack of a control group. Enrollment and clinical data collection were adversely impacted by the COVID-19 pandemic. Low power likely affected the ability to detect significant differences. For example, CF-related diabetes was associated with 22% increased risk of moderate periodontitis, but this was not statistically significant. Because CF is a rare disease, a multicenter research approach is necessary to recruit larger study populations with CF that would allow for adequately powered studies. Second, the study excluded adults with CF who were treated with steroids for pulmonary exacerbations in the last four weeks to avoid confounding. This affected the study by excluding less healthy individuals with advanced lung disease who experience frequent pulmonary exacerbations and biased the current study population toward healthier adults with CF. Future studies should make efforts to recruit adults with CF with varying degrees of lung disease severity. Third, this was a single-center study and while the population was representative of the US CF population in terms of CFTR genotype and race,1 most participants had a high income, completed four years of college, and had dental insurance. These factors biased the study population toward participants with low risk for periodontitis. To ensure representation of individuals of all socioeconomic levels in research, future studies should consider varying recruitment approaches. For example, future studies can utilize Medicaid or Medicare data to identify and recruit study participants in addition to the classic recruitment approaches (e.g., clinic or community-based recruitment).38 Lastly, the cross-sectional design study design did not capture variations in the examined risk factors over time (e.g., antibiotic use, diet). Longitudinal studies could help to definitively identify the risk of periodontitis in adults with CF.
CONCLUSION
Findings from this pilot study did not identify any condition-specific risk factors for periodontitis in adults with CF. However general risk factors such as age were associated with periodontitis in this population. Better powered longitudinal studies with larger sample sizes are needed to determine which condition-specific and general factors are associated with increased risk of periodontitis for this medically vulnerable population.
Footnotes
NDHRA priority area, Client level: Oral health care (prevention modalities).
DISCLOSURE
This work was supported, in part, by the National Center For Advancing Translational Sciences of the National Institutes of Health (Grant Number: UL1 TR002319), by the University of Washington Cystic Fibrosis Foundation Research Development Program (Grant Number: SINGH19R0), the National Institute of Diabetes, Digestive and Kidney Disorders Cystic Fibrosis Research Translation Center Clinical Core (Grant Number: NIH P30 DK089507), the National Heart Lung and Blood Institute of the National Institutes of Health (Grant Number: K23HL138154), the U.S. National Institute of Dental and Craniofacial Research (Grant Number: K08DE020856), the Dr. Douglass L. Morell Dentistry Research Fund, University of Washington School of Dentistry, the University of Washington Dental Hygiene Education Fund, and King Saud University. None of the funding sources were involved in study design, data collection, analysis, data interpretation, or writing of this manuscript.
- Received August 3, 2022.
- Accepted November 16, 2022.
- Copyright © 2023 The American Dental Hygienists’ Association