Abstract
Purpose: The emergence of SARS-CoV-2 has generated renewed interest in the potential transmission of viral contaminants via ultrasonic scaler-generated aerosols. The purpose of this study was to use controlled experimental conditions to quantify the range, direction, and concentration of aerosolized and splatter droplet spread across distances up to 106 inches from the source of the ultrasonic scaling procedure on a manikin patient head.
Methods: A dental simulation unit (DSU) was used to facilitate ultrasonic instrumentation performed on a typodont located within a manikin patient head. A 9 × 9-foot section of white paper was placed on the floor directly beneath the DSU. White paper was also placed on the adjacent countertops for identification of possible spread. Methylene blue dye was mixed with reverse-osmosis (RO) water and placed in the reservoir of the ultrasonic scaler. Experimental tests were run with high-volume evacuation (HVE) and a with a saliva ejector. Photographs of the paper and droplets were taken and analyzed by computer software to identify all droplets captured on the paper.
Results: Particle counts show that HVE use is associated with a reduction in total particle count for each zone evaluated, with the largest reduction seen in regions closest to the origin. Using HVE on the DSU demonstrated a 99% reduction in particles and 50% reduction in the range of particles.
Conclusion: Dental health care providers should use HVE when generating aerosols during ultrasonic instrumentation procedures to reduce particle spread in health care settings.
- aerosols
- ultrasonic scalers
- ultrasonic instrumentation
- high-volume evacuation
- saliva ejectors
- dental health care providers
Introduction
Aerosols are a common byproduct of many dental procedures, including ultrasonic scaling, tooth preparation with a dental handpiece, and the use of three-in-one air-water syringes.1 Existing evidence suggests that scaling and debridement procedures performed with ultrasonic instruments and water coolant produce the greatest aerosol and operatory contamination relative to other dental procedures.2, 3 Magnetostrictive and piezoelectric ultrasonic scalers typically oscillate between 20 – 42 kHz to remove plaque and calculus, as well as other potential aerosol contaminants with copious water lavage.4 However, ultrasonic scaling generates aerosols even in the absence of water, most likely due to the vibration of the insert.3
Numerous bacteria and viruses reside in the oral cavity and respiratory tract.1 These can be transported via aerosols, facilitating the spread of infectious diseases, including tuberculosis, pneumonia, influenza, and others.5 The 2019 emergence of SARS-CoV-2, also known as COVID-19, has sparked renewed interest in the potential transmission of viral contaminants in health care settings, with emphases on the production and spread of dental aerosols.
Prior studies have demonstrated that the greatest surface area of contamination from ultrasonic scaling procedures can be found within one foot of the operative site and detected up to four feet away.2 Cumulative contamination observed following ultrasonic scaling revealed that bacterial aerosols could be detected at a horizontal distance of 100 cm and a vertical distance of 50 cm from a patient’s oral cavity.6 However, these studies relied on “spot collection” wherein small sampling surfaces (filter discs, agar plates, or cassettes) were positioned at various locations throughout the examination room or operatory. While informative, given the small size of aerosolized particles (less than 50 microns)7 relative to the large footprint of a treatment room, such methods require extrapolating total particle dispersion from intermittent data points with many gaps. These studies intentionally avoided the use of high-volume evacuation (HVE) and did not explicitly test the effect of the suction at the source of contamination, the patient’s mouth. Without the use of HVE, these studies present a “worst case scenario” of droplet spread.2,6,7
Creation of a controlled experimental environment would allow for the range, direction, and concentration of aerosolized and spatter droplets to be measured and quantified during routine ultrasonic scaling. In an experimental environment, the use of the system’s HVE could be compared to the saliva ejector (SE) to assess their impact on particle spread and potential for each approach to facilitate infectious disease spread. The purpose of this study was to use controlled experimental conditions to quantify the range, direction, and concentration of aerosolized and splatter droplet (greater than 50 microns)7 spread across distances up to 106 inches (8.83 feet) from the source of the ultrasonic scaling on a manikin patient.
Methods
Experimental parameters and conditions
Two experimental tests were conducted in a large university dental simulation clinic equipped with all necessary dental equipment. No individuals were present beyond those directly involved with the study to limit the production of non-experimental aerosols. A dental simulation unit (DSU) (A-dec 41L; Newberg, OR, USA) equipped with a manikin head and face mask (Frasaco; Greenville, NC, USA) was positioned in full recline and situated such that the labial region of the mouth was 28 inches above the floor.
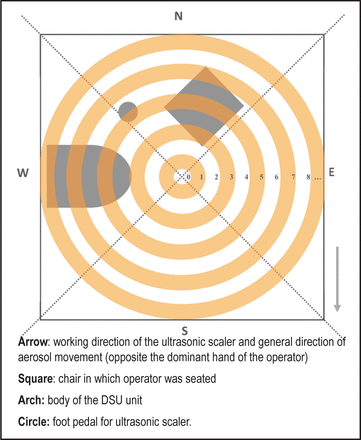
A large, continuous 9-foot x 9-foot section of white paper was placed on the floor directly beneath the DSU. A square, 8 x 8-foot perimeter was drawn onto the paper and the cardinal directions labeled to allow future digital orienting. A symbol was drawn immediately below the mouth of the DSU unit (hereafter referred to as “origin” or “zone 0”), as well as the footprint of the DSU unit, operator chair, and operator foot pedal (Figure 1). Additionally, two 48 x 18-inch sections of paper were placed on countertop surfaces beyond the floorplan directly across from the seated operator position. These served to detect any dispersion of particles beyond the 4-foot radial range of the floor paper. Both countertops were positioned 34.5 inches above the floor. One paper covered a countertop surface whose distance ranged from 51-69 inches from the origin, and the other 88-106 inches from the origin.
A quadrant system (not to scale) with concentric rings was used to photograph, organize and analyze the aerosol stain data. The zones consist of a series of nested concentric rings each situated with perimeters 3 inches apart (refer to Table I).
Zone and zone distance from origin
In order to visualize aerosolized particles and/or splatter, methylene blue dye was mixed with reverse osmosis (RO) water at a concentration of 0.5g dye to 500cc of water. This was added to the water reservoir of a magnetostrictive ultrasonic scaler. The operator, an experienced registered dental hygienist, wore protective eyewear, a mask with attached shield, a full coverage gown, and gloves, while performing the ultrasonic scaling procedures. Multiple temperature readings were taken prior to each experiment to ensure a consistent water evaporation rate would occur. The paper temperature was measured with an infrared video thermometer (CEM; Shenzhen Everbest Machinery Industry Co., Ltd, Shenzhen, China). The mean temperature for test one (SE) was 21.0°C (range 20.8 – 21.4) and for test two (HVE) was 20.1°C (range 19.9 – 22.0).
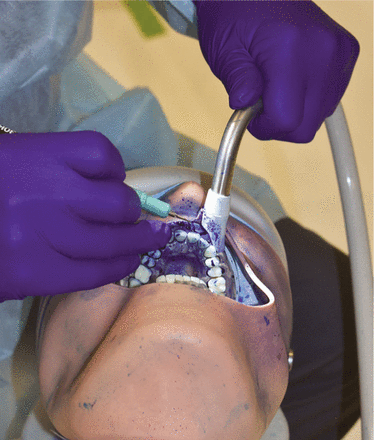
A 30 kHz magnetostrictive ultrasonic insert (Dentsply Sirona; Charlotte, NC, USA) was used for both tests. The power and lavage settings were set at 50%, which corresponded to a water flow rate of 18 mL/min as confirmed in prior studies. Both experiments required the operator to perform ultrasonic scaling on teeth numbers 6-11 (universal numbering system) for 5 minutes. A digital timer was used to record the time. The only difference between the two tests was the method of evacuation used: the first test used the system’s saliva ejector (SE) exclusively, while the second test used only the system’s HVE. Both evacuation systems were positioned and adjusted by the same experienced operator during the scaling procedures (Figure 2).
Dental simulation unit showing the operator performing ultrasonic scaling on teeth # 6-11 using HVE.
Since the airflow rates of the HVE and SE were expected to affect the study outcomes, airflows with the DSU and those in a nearby dental clinic were measured. Measurements made with a thermo-anemometer (Fieldmaster; Extech Instruments; Nashua, NH, USA) showed an average airflow of 0.58 ± 0.16 M/sec with the DSU’s HVE, which is considerably lower than the 2.17 ± 0.31 M/sec flow rate measured for the HVE in the clinic. Interestingly, the SE measurements for the DSU and the clinical units were comparable at 0.1-0.2 M/sec. At the conclusion of each testing cycle, all particle collection papers were allowed to sit undisturbed for 10 minutes to allow further aerosol dispersion and any stain-bearing particles settled on the paper to fully dry. Ten minutes was considered sufficient time to allow particles 7.4 um and larger to settle out and dry on the paper.8 The papers were moved, allowed to set for additional time, and prepared for imaging.
Data collection protocols
Each of the floorplans was overlain with a grid composed of 3 x 3-inch boxes. Each grid box was given a unique alphanumeric label that served not only as a landmark for photograph stitching, but also as a means to digitally orient each photograph in the subsequent composite images. The floorplan was systematically photographed using a digital single lens reflex camera and zoom lens (Nikon D3400, and AF-P DX NIKKOR 18-55mm, Nikon USA; Melville, NY, USA) with flash, on a tripod with a fixed position of 12 inches perpendicular to the floor.
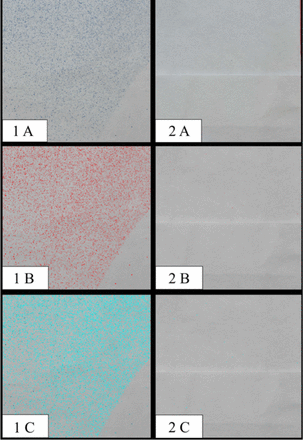
High quality images were imported into an imaging software Photoshop version 20.09 (Adobe; San Jose, CA, USA)xx0x0 where they were reoriented and merged to generate high resolution composite images reflecting large regions of the floorplan. The composite images were exported as uncompressed TIFs and imported into ImageJ9 where they were scaled and digitally thresholded to isolate the blue-stained particles (Figure 3). Regions-of-interest (ROIs) were manually created to isolate specific zones (Table I). Particle counts for each ROI were calculated using ImageJ’s native analyze particles feature. After analysis, the original image was compared to the digital particle rendering and data points reflecting any obvious non-stain particles (e.g., hair, dust, debris) were identified. These, along with any particle whose circularity index was less than 0.5 (i.e., particles with a highly linear profile), were excluded from all subsequent analyses. These conservative measures ensured isolation of true aerosolized and splatter particles.
Image series showing the reduction in detectable particle concentrations on the floor while ultrasonic scaling using a SE (left) and while using HVE (right).
Each square represents a commensurate 3 x 3-inch section taken from the southern quadrant of the floor (4.5-7.5 inches from origin).
Note: The curved outline of the operator’s shoe is visible in images reflecting SE use only, with evidence that the foot was moved part-way through the test.
A: Raw image showing blue-stained particles
B: Particles isolated after digital thresholding
C: Particles identified and subsequently quantified for analysis.
Results
Particle dispersion
In total, 166,137 particles were identified for test one (SE only) and 1,655 for test two (HVE), indicating an overall reduction of 99% with the use of HVE (Figure 4). The furthest zone with detectable particles when using HVE was zone 8 (22.5 - 25.5 inches), nearly half the distance seen when using an SE only (zone 15, or 43.5 - 45.6 inches) (Table II). Neither test was associated with spread of aerosols beyond 4 feet as evidenced by the fact neither counter surface possessed detectable aerosol staining after the wait time. Anecdotally, aerosol staining was seen on the operator face mask and face shield following both tests, however this was not quantified.
Particle count by quadrant. Saliva ejector (SE) (Red Orange) and High-volume evacuation (HVE) (Yellow orange).
Differences in scale for particle count: SE: 0 – 30,000, HVE: 0 – 300.
Particle count by zone.
Particle counts show that HVE use is associated with a reduction in total particle count for each zone evaluated, with the largest reduction seen in regions closest to the origin and the smallest in regions beyond zone 7 (Table II). There was a 340-750% reduction in particle count in zones immediately below and adjacent to the manikin’s mouth (Figure 3).
Test one (SE) shows the highest concentration of particles in both the southern (45% particles) and eastern (44% of particles) quadrants, which were situated opposite to the working hand of the operator in the expected direction of fluid flow (refer to Figure 1 for quadrant and floorplan orientation). Particle rates were low in the northern (10%) and nearly absent in western quadrants (< 1%), to the right and towards the feet of the patient/manikin, respectively. As seen in Figure 3, particle count distributions in each quadrant decreased with increasing distance, with zones closest to the origin having a higher number of particles despite these zones being smaller in total size.
In test two (HVE), the highest particle concentration was seen in the eastern quadrant (66%), followed by the western (21%), southern (10%) and northern (3%) quadrants. In addition to having fewer particles overall, the distribution of particles differs when using HVE (compared to the SE) in that there was not a linear decrease in particle count with increased distance. Rather, the particle distribution within each quadrant roughly followed a bell curve, with particle counts peaking in zones 3-5 (7.5 – 16.5 inches from origin), though counts for the eastern quadrant are higher and more variable across zones. This may indicate that use of HVE is disproportionately effective at capturing particles in zones situated less than 17 inches from origin.
Discussion
Results support the use of HVE to effectively reduce the total spread of both splatter and aerosolized droplets that exit a patient’s oral cavity during ultrasonic scaling procedures. Previous research shows such particles routinely transport viruses, blood, and supra- and sub-gingival dental plaque6 creating an avenue for infectious disease transmission in the absence of proper personal protective equipment and aerosol mitigation methods such as HVE.
High concentrations of bacterial aerosols have been identified by culturing colony-forming units from the patient following ultrasonic scaling procedures.10 Importantly, the use of high-volume evacuation during ultrasonic scaling on human subjects reduces bacterial spread as evidenced by a lower number of colony-forming units on blood agar plates placed in the treatment operatory during the procedure.11 Evidence of viral transmission via splatter and aerosol is more limited; however, it is known that viral agents can be carried by aerosolized body fluids depending on the size of the viral agent, the transporting particle, and certain environmental conditions including relative humidity and temperature.12, 13 While the assessment of bacterial and viral agents were beyond the scope of this study, results indicate that the application of HVE likely reduces the spread of disease by limiting the spread of potentially infectious fluids.
The results of this study also show a significant reduction in settled particles detected following ultrasonic scaling with HVE compared to the exclusive use of a SE. These findings corroborate work by Jacks,14 who found a 90% reduction in the concentration of particles created by an ultrasonic scaler with the use of HVE compared to a SE alone. These results are significant, as dental hygienists in private practices and dental clinics often work independently without the aid of a dental assistant. As a result, they may be less likely to employ HVE during ultrasonic scaling procedures.
Based on these findings and those of other related studies, clinicians working without a dental assistant should make every effort to use some form of HVE rather than relying exclusively on the SE. One promising way to allow simultaneous ultrasonic instrumentation and HVE by a single operator would be an HVE attachment with an integrated mirror or mouth pieces coupled with an HVE attachment. However, more research on the degree to which such systems also reduce particle spread and concentration are needed, in addition to research on the practicality of such systems for dental hygienists.
The greatest particle concentrations following ultrasonic scaling were identified in the southern and eastern regions surrounding the patient, which in these experiments, were situated directly opposite the working arm of the operator, in the general direction of water flow from the ultrasonic scaler (Figure 2). This is consistent with prior research showing the greatest surface area contamination following ultrasonic scaling was found between the four and six o’clock positions of the patient’s head (equivalent to the southern region in this study).2 Similar to this study, no contamination was found at distances over four feet from the patient’s oral cavity.2
Results of this study support the conclusion that a dental health care provider may reduce their exposure to splatter and aerosols by at least 99% by using HVE. The DSU used in this study had an HVE air flow rate that was approximately 25% of that found in a clinical chair. It is reasonable to assume that a clinical HVE unit would provide an even greater reduction in the number of particles detected. Eliminating aerosols and splatter at the source will also limit the production and spread of particles and downstream contamination risk. Importantly, the biggest reduction in particle count with HVE was seen in zones closest to the operator and dental assistant (i.e., less than 2 feet from the source). This suggests that the biggest benefit to using HVE is for those proximate individuals most at risk - the dentist, dental hygienist, and dental assistant.
In addition to HVE use, preprocedural mouth rinses can also reduce the risk of infectious disease transmission at the source, decreasing the number of bacterial and viral agents present in the oral cavity and limiting the production of contaminated aerosols. Chlorhexidine gluconate is an effective preprocedural antibacterial mouth rinse, reducing 61% - 93% of bacteria detected on blood agar plates following dental prophylaxes.15, 16 Furthermore, a 0.2% chlorhexidine gluconate pre-procedural mouth rinse used in conjunction with HVE has demonstrated significantly greater reduction of contaminated aerosols than a pre-procedural mouth rinse or HVE alone16. One percent hydrogen peroxide and 0.2% povidone iodine have been recommended as potentially effective against SARS-CoV-2 due to the susceptibility of the virus to oxidation,17 with viral inactivation within 15 seconds when using 0.5%, 1%, 1.5% povidone iodine mouth rinses.18 While more research is needed, early results suggest that incorporating a combination of HVE and pre-procedural rinses into dental hygiene best practices will significantly reduce the risk of patient-to-dental health care provider viral and bacterial disease transmission. This is particularly true when added to the existing use of personal protective equipment, cleaning and sterilization practices, and engineering protocols (e.g., barriers).
This study had limitations. The composition and contents of the aerosol droplets produced in-vivo were not studied in this set of in-vitro experimental tests. Particulates such as bacteria, viruses, and other organic and inorganic material contained in clinically produced aerosols were not examined. Future clinical studies may further clarify components of aerosol droplets produced during ultrasonic instrumentation. In addition, the size of the airborne droplets was not measured, but rather the size of the spots left after the drops landed on the white paper. Droplets were measured according to post-splatter size, which may have been larger than the droplets expelled from the ultrasonic scaler.
Conclusion
This study addressed the importance of adding critical engineering equipment controls to reduce dental health care providers exposure to potentially infectious materials. Results of this study demonstrate that use of HVE can reduce splatter and aerosol production by up to 99% and reduce the distance that particles disperse by up to 50%. The broader clinical relevance of these results is twofold: use of HVE reduces potential disease exposure to dental health care providers, and the use of HVE restricts the range that particles may spread (e.g., to nearby operatories or examination rooms). High volume evacuation systems should be used in conjunction with other mitigating controls for all aerosol-generating dental procedures.
Acknowledgments
The authors would like to thank Megan Davis, Arlene Bergner, Vivian Jauregui, Laura Scott, and other clinical staff members who helped with data collection and processing and offered helpful recommendations for the project design.
Footnotes
This manuscript supports the NDHRA priority area Client level: Basic science (diagnostic testing and assessments).
Disclosure
No external funding was received for this project. The authors have no conflicts of interest to report.
- Received August 10, 2020.
- Accepted November 3, 2020.
- Copyright © 2021 The American Dental Hygienists’ Association