Abstract
Purpose: To investigate the anti-gingivitis efficacy of a novel oral hygiene routine consisting of a two-step stannous fluoride dentifrice and hydrogen peroxide whitening gel system, an interactive oscillating-rotating electric toothbrush, and expanded polytetrafluoroethylene floss.
Methods: A total of 52 participants (n=52;mean age 35.8±11.23 years) were enrolled in the study and randomized 1:1 to the experimental hygiene group or control (dental prophylaxis followed by use of standard sodium fluoride dentifrice and a manual toothbrush). Participants were instructed to brush twice daily; those in the experimental group were instructed to floss once daily. Oral examinations were conducted at Baseline, Week 2, Week 4, and Week 6.
Results: Both groups experienced significant declines in the mean number of bleeding sites from Baseline at all time points, evident as early as Week 2. Bleeding sites continued to decline throughout the trial in the experimental group, whereas they showed an increasing trend between Weeks 2 and 6 in the control group. The experimental group had 55% fewer bleeding sites at Week 2, 85% fewer bleeding sites at Week 4, and 98% fewer bleeding sites at Week 6 (p<0.0001 for all) as compared to the control group. At Week 6, 84% of participants in the experimental group had no bleeding, while all participants in the control group had bleeding.
Conclusion: The experimental oral hygiene group showed significantly greater reductions in gingival bleeding than the control oral hygiene group, with benefits seen as early as Week 2 and increasing over the six-week study.
Introduction
Gingivitis is characterized by inflammation of the gingival tissues without loss of connective tissue attachment.1 The disease progresses when oral bacteria present in dental plaque prompt a localized inflammatory response manifesting as gingival redness, swelling, and bleeding.2 Persistent gingivitis is one possible risk factor for periodontal attachment loss as well as tooth loss.3,4 Considering that over 90% of American adults exhibit signs of gingivitis of at least mild severity,5 advancements in treatment are an important public health concern. The correlation between dental plaque and the severity of gingival disease is well understood.6,7 Therefore, in addition to regular professional dental prophylaxes, a cornerstone of gingivitis treatment is rigorous daily removal of dental plaque through both mechanical means (e.g., brushing, flossing) and chemotherapeutic means (e.g. anti-plaque chemical agents in a mouth rinse or dentifrice).8
One advancement in mechanical dental plaque removal for gingivitis prevention is the use of rechargeable electric toothbrushes, which have been shown to reduce plaque accumulation more effectively than manual toothbrushes.9 Among the electric toothbrush modes of action, oscillating-rotating toothbrushes have been found to reduce plaque and gingivitis more effectively than side-to-side brushes in short-term trials.10,11 Recently, interactive electric toothbrushes that communicate with an application on a smart phone have been shown to be associated with significantly longer brushing times, a greater extent of plaque reduction, and higher compliance rates as compared to manual toothbrushes.12,13 This last benefit is of key importance, given that many adolescents and adults are non-compliant with their recommended oral hygiene routine.14 Classic studies indicate that adults generally overestimate the time they spend brushing by 50 to 70 seconds.15 Various factors may influence patient compliance including patient characteristics (beliefs and attitudes, history of noncompliance, mental and physical disabilities); treatment complexity and duration; the relationship between the patient and provider; and behavioral interventions used (praise, education interventions).16 Interactive electric toothbrushes may increase patient compliance by acting upon several of these factors. For example, an application on a smart phone may cause the patient to feel that the oral hygiene routine is easy to perform, and the positive feedback and education provided by the application may serve as positive behavioral interventions.
Beyond brushing, interdental mechanical plaque control is an additional strategy for the treatment of gingivitis. Various interdental cleaning devices include dental floss, interdental brushes, and irrigators. One specific device, expanded polytetrafluoroethylene floss, has been shown to provide benefits for gingivitis treatment when used alone, and further incremental benefits seen when used with brushing.17 More importantly, subjects have been shown to prefer expanded polytetrafluoroethylene floss over nylon waxed floss, which may contribute to improved compliance.18
The addition of chemotherapeutic agents, such as the antimicrobial chlorhexidine, to the oral hygiene routine is another strategy for gingivitis prevention and treatment.19 Despite its effectiveness for gingivitis treatment, chlorhexidine has been associated with tooth staining20, making it less acceptable for use. Recently, a two-step stannous fluoride dentifrice and hydrogen peroxide whitening gel system was shown to provide gingival health effects comparable to those seen with a chlorhexidine mouth rinse, with tooth whitening effects.21,22 A meta-analysis of 1085 subjects enrolled in 20 prospective trials in which one group was assigned to the two-step system and another group to a standard dentifrice control found the two-step system was associated with significant improvements in plaque measurements and gingival bleeding versus the control.23
The purpose of this study was to investigate the anti-gingivitis efficacy of a novel oral hygiene routine consisting of a two-step stannous fluoride dentifrice and hydrogen peroxide whitening gel system, an interactive, oscillating-rotating electric toothbrush, and expanded polytetrafluoroethylene floss as compared to a control group.
Methods
This randomized, controlled, examiner-blind, clinical trial evaluated the effect of an experimental oral hygiene routine consisting of a two-step stannous fluoride dentifrice and hydrogen peroxide whitening gel system (Crest® Pro-HealthÔ [HD]; Procter & Gamble, Cincinnati, OH, USA), an interactive rechargeable electric toothbrush (Oral-B® Professional Care SmartSeries 5000 toothbrush with Oral-B CrossAction® toothbrush head, D36/EB50, Procter & Gamble), and an expanded polytetrafluoroethylene floss (Oral-B® Glide® Pro-Health Advanced, Procter & Gamble) as compared to a control group receiving a an oral prophylaxis and using a standard sodium fluoride dentifrice (Crest® Cavity Protection, Procter & Gamble) and soft manual toothbrush (Oral-B® Indicator, Procter & Gamble), on gingival bleeding over a 6-week period in subjects with mild-to-moderate gingivitis. Institutional review and approval was obtained from Nova Southeastern University; approval #2016-209. The study was conducted in compliance with the International Conference on Harmonization's Good Clinical Practice Consolidated Guidelines. All participants provided written, informed consent.
Participants
Eligible participants were 18 years of age or older, in good general health, owned a smart phone to which they were willing to download the Oral-B application. The application provided coaching for the 2-minute brushing time. Subjects were specifically instructed on the pressure alert feature to promote brushing with proper force. Eligible subjects had at least 16 gradable teeth, and at least one anterior and one posterior facial bleeding site. Exclusion criteria included severe periodontal disease; active treatment for periodontitis; fixed facial or lingual orthodontic appliances; or antibiotic use within two weeks of Baseline.
Study Design
Participants were randomly assigned in equal numbers to either the experimental group consisting of 6 weeks of using three marketed oral hygiene products (an interactive rechargeable power toothbrush, two-step dentifrice/whitening gel sequence, and floss) or the control group receiving a full-mouth dental prophylaxis administered within 3 days of Baseline, followed by 6 weeks of using standard oral hygiene products (a regular manual toothbrush and standard anti-cavity dentifrice). Participants were stratified by number of bleeding sites (high ≥10, medium 6-9, low ≤5). Within strata, participants were randomly assigned to one of the treatment groups using an encoded program and randomization schedule supplied by the study sponsor. The treatment code was shared with one of the site staff members to allow for identification of participants that were to undergo dental prophylaxis (control group).
All participants were instructed to use the study products in place of their usual oral hygiene products for the duration of the 6 week trial; all participants were verbally instructed in the use of the study products. The first use of the study products was supervised. Written instructions appropriate to the group assignment were provided to each participant. Experimental group participants were instructed to brush their teeth twice daily using the “Daily Clean” mode on the brush and to floss the whole mouth once daily. Participants were instructed to follow manufacturer's instructions for the brushing technique. In regards to the toothpaste, participants were instructed to brush with the first step of the 2-step sequence (stannous fluoride dentifrice) for one minute and then brush with the second step (hydrogen peroxide whitening gel) for the second minute, according to manufacturer's instructions. Control group participants were instructed to brush twice daily according to their customary brushing manner. Participants were instructed not to use other dental hygiene products for the duration of the study.
Investigational Products and Blinding
All study related products and instructions were supplied by the study sponsor in identically sized, blinded kit boxes. The identities of the dentifrices and dental floss in the kit boxes were blinded. The identity of the electric toothbrush provided to the experimental group was not blinded.
Assessments and Outcomes
Dental examinations, including examination of the soft and hard oral tissues and gingival exams, were conducted at Baseline, Week 2, Week 4, and Week 6 by a trained, experienced examiner.24-26 Assessment of the oral soft tissue was conducted via a visual examination of the oral cavity and perioral area utilizing a standard dental light, dental mirror, and gauze. Assessment of the oral hard tissues was conducted via a visual examination of the dentition and restorations utilizing a standard dental light, dental mirror, and air syringe.
The primary efficacy outcome was gingival bleeding, which was assessed across the whole mouth.26,27 This method used mild provocation of the gingival crevice with a periodontal probe at 2 mm depth passed gently circumferentially around each tooth at approximately a 60° angle. After 30 seconds, each tooth site was assessed for bleeding. Using this clinical method, bleeding sites were derived from using the 4-point Löe-Silness gingivitis index (LSGI) for sites with LSGI ≥ 2.28 The full mouth bleeding score was determined by summing the bleeding scores of all scored sites.
Adverse Events
An adverse event (AE) was defined as any unfavorable or unintended sign, symptom, or disease that appeared or worsened in a participant during the study period. AEs were collected from examination and interview.
Statistical Methods
Up to 52 subjects were to be enrolled in the study; 26 per group. Twenty-three subjects per group completing the trial provides at least 85% power to detect a mean difference between Baseline and Week 6 of at least 4 bleeding sites using two-sided testing at a 5% significance level. This estimate assumes the standard deviation of the differences between Baseline and Week 6 is six bleeding sites or smaller. Summary statistics of the demographics and number of bleeding sites were calculated for each treatment group and visit. Group differences for age and baseline number of bleeding sites were compared using Analysis of Variance. A Chi-Square test was used to assess gender balance between the two groups while a Fisher's Exact test was used to assess ethnicity balance. Comparisons to baseline were investigated using paired-difference t-tests. The treatment groups were compared using the analysis of covariance method with baseline as a covariate and a baseline by treatment interaction. Different variances were modeled for each treatment. Statistical tests were two-sided using a 5% significance level.
Results
Participant Baseline Demographics and Clinical Characteristics
A total of 52 participants were enrolled in the study and randomized 1:1 to the experimental group or the control group (Table I). One subject voluntarily withdrew so 51 subjects (n=51) completed the trial. Participants ranged in age from 19 to 60 years, with a mean age of 35.8±11.23 years. There were more females (n=37) than males (n=15) in this study (71% vs. 29%). There were no significant differences between groups at Baseline for age, ethnicity, sex, or number of bleeding sites.
Number of Bleeding Sites
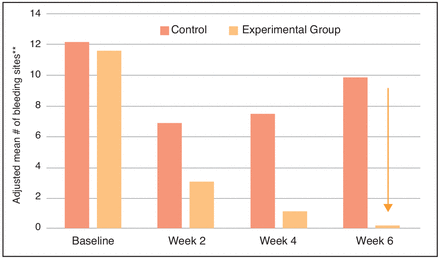
Both groups experienced significant declines in the mean number of bleeding sites from Baseline at all time points, and these declines were evident as early as Week 2 (Figure 1). Of note, the number of bleeding sites in the experimental group continued to decrease throughout the trial, whereas after Week 2, the control group showed an increasing trend. At Week 2, the change from Baseline in the mean number of bleeding sites and (SD) was -5.0(5.1) in the control group and -8.5(5.9) in the experimental group (p<0.0001 for both compared with Baseline). At Week 4, the change from Baseline in the mean number of bleeding sites (SD) was -4.7(3.9) in the control group, and -10.8(5.8) in the experimental group (p<0.0001 for both compared with Baseline). At Week 6, the change from Baseline in the mean number of bleeding sites (SD) was -2.0(3.7) in the control group (p=0.0127) and -11.4(6.3) in the experimental group (p<0.0001). Bleeding site trends are shown in Figure 1.
Baseline demographics and clinical characteristics
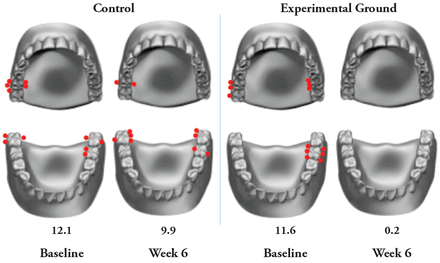
Location of bleeding sites per group at Baseline and Week 6.
The experimental group had statistically significantly fewer bleeding sites than the control group in the direct comparison for number of bleeding sites (Figure 2). Compared to the control group, the experimental group had 55% fewer bleeding sites at Week 2, 85% fewer bleeding sites at Week 4, and 98% fewer bleeding sites at Week 6, which were all highly significant differences (p≤0.0001).
Percent of Participants with No Bleeding
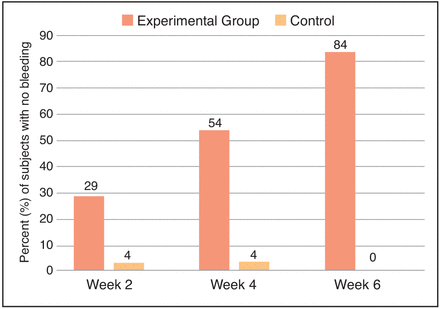
At Week 2, 29% of the participants in the experimental group exhibited no gingival bleeding, as compared to only 4% in the control group. By Week 4, the experimental group continued to improve, with 54% exhibiting no gingival bleeding, while the control group remained unchanged. After 6 weeks, 84% of participants in the experimental group had no bleeding, while all participants in the control group had gingival bleeding (Figure 3).
Safety
There were no AEs reported at any time point.
Discussion
Results of this six-week study demonstrate that an experimental oral hygiene routine consisting of a two-step stannous fluoride dentifrice and hydrogen peroxide whitening gel system, an interactive oscillating-rotating electric toothbrush, and expanded polytetrafluoroethylene floss, was significantly more effective at reducing gingival bleeding when compared to a control oral hygiene routine of a professional dental prophylaxis, followed by the use of standard sodium fluoride dentifrice and a soft manual toothbrush. As shown in Figure 1, bleeding sites were most prevalent in the posterior region, an area that can be difficult for patients to access and thereby at higher risk for gingivitis.29 Notably, the reductions in the mean number of gingival bleeding sites seen in the experimental group were evident early in the trial, after only 2 weeks of use, and increased in magnitude over the course of the 6-week study. In the control group, reductions in gingival bleeding were also seen at Week 2, likely due to the dental prophylaxis at Week 0. However, the long-term benefits were not as great in the control group. The reduction in the mean number of bleeding sites compared with Baseline was smaller at Week 4 and Week 6 than at Week 2. The greater long-term reduction in gingival bleeding seen in the experimental group versus the control group indicates that effective daily oral hygiene is important to prevent reoccurrence of bleeding.
Number of bleeding sites per group.
*Statistically significant difference between groups in favor of the experimental group, p≤0.0001.
**Based on Analysis of Covariance. Baseline values are means.
Percent of participants with no gingival bleeding.
This trial evaluated the effect of a combination of products, representing typical oral hygiene practices, and therefore conclusions cannot be drawn about the specific contribution of each individual product to gingivitis outcomes. However, previous studies have shown that the addition of a stannous-containing fluoride dentifrice with an power toothbrush significantly increased plaque control compared to a standard sodium fluoride toothpaste with the same electric toothbrush.30 These findings indicate there is an incremental benefit when effective chemotherapeutics are added to mechanical hygiene. While assessing compliance was not an objective of this trial, the interactive power toothbrush has been shown to increase brushing time relative to a manual toothbrush among adolescents.13 It would be an interesting topic for future research to assess compliance of a product combination including the interactive toothbrush.
The most noteworthy limitation of this clinical trial involves the study population. This research was intended to be inclusive, and as such, targeted a general population. Subjects for this study generally presented with mild-to-moderate gingivitis, as evidenced by the overall mean of approximately 12 bleeding sites prior to prophylaxis. Severe disease was underrepresented, and further research may be indicated to ascertain responses in other patient types. Study duration was 6-weeks post-prophylaxis, and although trends were clear, long term implications may warrant further investigation. Inference is likely most relevant to the short-to-intermediate duration responses seen with regular recall subjects.
When examining the percentage of participants with no bleeding sites, the experimental product combination was again more effective than the control at all time points. After 6 weeks, 84% of participants in the experimental group were completely free of bleeding sites, compared to none of the participants in the control group. These results are clinically relevant given that the control group received a baseline dental prophylaxis, which is considered the ‘gold standard’ treatment for gingivitis, and by Week 6 all subjects in the control group exhibited gingival bleeding again. Based on these findings, oral healthcare professionals should consider the products in the experimental group for subjects with mild-to-moderate gingivitis to reduce their gingival bleeding and inflammation, thereby improving their periodontal health.
Conclusion
This randomized clinical trial was conducted to investigate the anti-gingivitis efficacy of a novel oral hygiene routine consisting of a two-step stannous fluoride dentifrice and hydrogen peroxide whitening gel system, an interactive, oscillating-rotating electric toothbrush, and expanded polytetrafluoroethylene floss. Study results demonstrated significantly greater reductions in gingival bleeding for the novel oral hygiene routine as compared to the control oral hygiene routine comprised of a professional dental prophylaxis followed by the use of standard fluoride dentifrice and a manual toothbrush. Benefits for the experimental hygiene group were demonstrated as early as Week 2 and increased over the six-week study. At Week 6, the experimental group had 98% fewer bleeding sites than the control group. Thus, the novel oral hygiene routine was shown to have effective and sustained anti-gingivitis efficacy.
Acknowledgements
Editorial assistance to the authors was provided by Jillian Lokere, MS, and was funded by Procter & Gamble Company. Statistical support was provided by Melanie Miner, BS, BA.
Footnotes
Cristina E. Garcia-Godoy, DDS, MPH is a clincal researcher; Kevin L. Flores, BS, MPH is a research assistant; both at Nova Southeastern University, Ft. Lauderdale, FL.
Malgorzata A. Klukowska, DDS, PhD is a principal clinical scientist in reserch and development; Erinn L. Conde, BS is a clinical trial manager; Robert W. Gerlach, DDS, MPH is a retired research fellow; all at Proctor and Gamble, Mason, OH.
This manuscript supports the NDHRA priority area: Client level: Basic science (Diagnostic testing and assessments).
Disclosure
This study was funded by Procter & Gamble. Dr. Klukowska and Ms. Conde are employees of Procter & Gamble and Dr. Gerlach is a retired employee of Procter & Gamble. The other authors have no conflicts to disclose.
- Received February 2018.
- Accepted August 28, 2018.
- Copyright © 2018 The American Dental Hygienists’ Association