Abstract
Purpose: The American Association of Poison Control Center's annual reports demonstrate that acute fluoride exposure is not an uncommon occurrence. Despite its prevalence, there has been little published research on the topic in the last 10 years. The purpose of this study was to calculate the incidence of acute fluoride toxicity and lethality as it occurs in New Jersey and provide a descriptive epidemiology of acute fluoride exposures.
Methods: The study design was retrospective in nature. Records of phone calls made by individuals reporting excessive fluoride exposure (in an amount greater than directed/prescribed) to New Jersey's poison control center, known as Poison Information and Education System from the years 2010 through 2012, were extracted from Toxicall® (Computer Automatic Systems, Inc.) database. A total of 2,476 human-only exposure records met the inclusion criteria and were analyzed. Incidence rates were calculated, and population characteristics, circumstances and medical outcomes of acute fluoride exposure cases were assessed and categorized.
Results: A total of 2,476 phone call records met the inclusion criteria. The fluoride exposures reported were from toothpaste with fluoride (49%, n=1,214), mouth rinse with fluoride (21.6%, n=536), multivitamin with fluoride (21.4%, n=530) and pure fluoride (0.08%, n=199). Medically speaking, 94.75% of calls were asymptomatic cases (n=2,346), 4.24% were symptomatic (n=105) and 1.01% were informational inquiries (n=25). Adverse symptoms reported were mostly minor (83.9% of symptomatic cases, n=88) and moderate (16.1% of symptomatic cases, n=17). The age group 18 months to 3 years of age showed the highest incidence of acute fluoride exposure (53.2%, n=1,317). There was a slightly higher incidence of acute fluoride exposures among males (n=1,317) vs. females (n=1,159). Most incidences occurred in the home (93.1% of records, n=2,305) and occurred unintentionally (96.7%, n=2,394). Calls were mainly made by the subject's mother (67.5%, n=1,671).
Conclusion: Based on the data, there were no reports of lethality or toxicity due to acute fluoride exposure in New Jersey from 2010 through 2012. Symptomatic reports and informational inquiries were few. All adverse outcomes due to excessive fluoride intake were remedied with calcium as the antidote. Dental hygienists should educate patients on safety measures of fluoride-containing products and evaluate overall fluoride exposure prior to making recommendations. However, findings in this study suggest that levels of fluoride in available commercial products will not produce life-threatening events, even if taken in doses higher than recommended.
Introduction
The introduction of fluoride as a preventative measure against tooth decay dates back to the early 20th century, during a time when dental caries were ubiquitous among children of all classes.1 Today, fluoride is considered, by many, the best defense against dental caries. Fluoridation of water was named by the Centers for Disease Control and Prevention (CDC) as 1 of the 10 most important public health measures.2 There has been significant support for dental products with fluoride, including toothpaste, mouthwash, multivitamins, dietary supplements and in-office treatments.3 With the combination of topical and systemic fluoride, a decline in caries has been seen globally.4 Studies also show that the benefits of fluoride are lifelong and not restricted to children with developing teeth.5
Despite the benefits of fluoride, there is a potential for harm resulting from chronic and acute exposure to fluoride. Chronic exposure to fluoride can lead to fluorosis, which is systemic in nature and caused by disruptions in enamel formation that occur during tooth development.4 Long-term exposure can also cause crippling skeletal fluorosis, which is characterized by increased density of bone (osteosclerosis) and the formation of bony outgrowths.6
Acute fluoride poisoning is contingent upon several factors and can cause a variance of symptoms. When products are used in the volumes or weights indicated, there is usually little danger of serious, systemic acute toxicity. However, when topical gels are applied to small children incorrectly or ingested in quantities that exceed recommended doses, symptoms of toxicity and potential for serious toxicity is present.7 Acute ingestion of fluoride can lead to nausea and gastrointestinal irritation. Large amounts of ingestion of fluoride can lead to organ damage and even death.8
Acute fluoride toxicity depends not only on the amount of fluoride intake but the patient's weight.4 Children tend to be more susceptible to harm from fluoride toxicity than adults. The dose-response relationship is important to understand that health response is chemical, dose and organ specific.4 The values of acute fluoride toxicity can be seen in Table I.9 An average 2-year-old child weighing 30 pounds would require 67 mg of fluoride to reach the acute toxic dose, and an adult weighing 180 pounds would require 400 mg.
As fluoride is a drug, the U.S. Food and Drug Administration (FDA) is responsible for approving prescription and over-the-counter fluoride products in the U.S. and for setting standards for labeling.16 The amount of fluoride permitted in dental products is under the ongoing regulatory authority of the FDA to prevent fluoride toxicity. The American Dental Association (ADA) sets criteria for products to gain the voluntary ADA Commission on Scientific Affairs Seal of Acceptance, which is in compliance with the FDA regulations. To meet FDA regulations, over-the-counter toothpastes must have less than 276 mg F per tube.21 If needed for therapeutic reasons, toothpastes containing more fluoride are available but usually obtained only with a prescription. The amount of fluoride contained in a dental product is sometimes given as a percent of volume or in “parts per million” fluoride (ppm F) in the labeling to make it more consumer relatable.21
Most current research on fluoride toxicity has focused on chronic exposure. There are a limited number of publications on acute fluoride toxicity, despite its common occurrence as demonstrated by the national-based American Association of Poison Control Center (AAPCC). According to the AAPCC National Poison Data System's (NPDS) 29th report (2011), 30,000 calls regarding excessive fluoride exposure were made to poison control centers across the nation.10 The report reveals that most acute fluoride exposures were in children 5 years and younger. Almost all of the cases had no medical outcomes, however, there were a couple cases resulting in moderate and major adverse medical outcomes, such as major gastrointestinal symptoms, and indirect deaths.10 Although statistics about fluoride overexposure as reported to poison control centers across the nation is published in AAPCC's annual report, the specific widespread issue is not explored or analyzed further. The lack of recent data in literature has undermined the importance to study and analyze current trends in acute fluoride exposure. Fluoride plays a prominent role in current preventative practices against caries; therefore, it is important oral health care professionals remain current on the topic.
A study of fluoride toxicity is also important to help in light of recent controversies in the media regarding the safety of fluoride. While numerous studies establish a causal relationship between fluoride and the prevention of dental caries,3 anti-fluoride proponents argue that fluoride is a “potent poison.”11 They argue that the warning label on fluoridated products required by the FDA (as is for all drugs under its regulatory authority) is reason to believe that fluoride is dangerous.16 The label states: “If more than recommended is accidentally swallowed, get medical help or contact a poison control center right away.”16 Anti-fluoride proponents also use the fact that there are thousands of calls made to poison control centers every year as a result of excessive ingestion of fluoride, “many of which result in emergency treatment at a medical facility” as evidence to support their claims.11
The ADA, however, states that most media coverage has not revealed that the ADA limited the amount of fluoride allowed in ADA-accepted dentifrices years ago. To reduce the likelihood of accidental poisoning among children, the ADA requires that no more than 120 mg of fluoride, or 264 mg of sodium fluoride, be dispensed in one container of fluoride rinse, gel or supplement.8 This is less than what is mandated by the FDA (which is 276 mg F).9 The CDC and ADA encourages practitioners to evaluate all potential fluoride sources and conduct a caries risk assessment prior to prescribing fluoride supplements. ADA argues that the warning label “greatly overstates” any danger posed by fluoridated products.13
The purpose of this study was to explore the issue further to unearth some of these controversies and update current literature on acute fluoride exposures. An additional purpose was to calculate the incidence of acute fluoride toxicity and lethality as reported to New Jersey's Poison Control Center. The study will follow a descriptive epidemiological format to provide insight on commonly affected groups and medical outcomes of excessive fluoride exposure.
For purposes of this study, acute exposure/excessive exposure is defined as the amount taken to be greater than what has been prescribed, suggested or thought to be normal. This is not necessarily a toxic or poisonous amount. This can include cases of acute on chronic exposure, but not chronic exposure alone.
Methods and Materials
The research design is a retrospective cohort study. Collaborative Institutional Training Initiative training was completed by all investigators. The study obtained institutional review board approval and funding was provided by Rutgers School of Public Health Exploratory Grant Program. Records of phone calls made by individuals reporting excessive fluoride exposure to New Jersey Poison Information and Education System were extracted from Toxicall electronic database.
New Jersey Poison Information and Education System is the regionally certified poison center in the state of New Jersey that receives calls related to fluoride exposure from all 21 counties. Toxicall is used at New Jersey Poison Information and Education System to collect and record data on calls made to the center regarding possible poisoning and over-exposure to substances, in addition to any questions related to medical substances, chemicals, foodborne illness, etc. The trained Specialist in Poison Information (SPI) with a background in pharmacology answers calls made to New Jersey Poison Information and Education System and collects as much information as possible about the suspected overexposure. This information includes date and time of call, type of substance, patient's age and gender, reason for exposure, county of caller, caller's zip code, relationship of caller to patient, location of exposure, and medical outcome of exposure (none, minor effect, moderate effect, major effect or death). Medical advice is provided according to the details of the case presented. The SPI handling the call documents all pertinent data and enters it into the database in accordance to the poison control center coding handbook. For fluoride exposure, the SPI determines toxicity based on a calculation to determine the ratio of mg/kg. For example, a child who ingested 50 tablets of 0.25 mg fluoride with a weight of 11.36 kg has ingested 1.1 mg/kg fluoride. To demonstrate: 50 tablets × 0.25 mg/tablet = 12.5 mg NaFl / 11.36 kg = 1.1 mg/kg NaFl
Inclusion/Exclusion Criteria of Data for Study
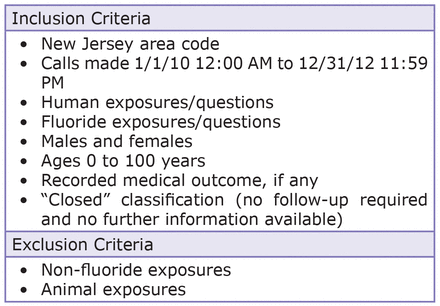
Figure 1 lists the inclusion and exclusion criteria of the extracted data. Classifications were deemed and coded by SPI's. No personal identifying information was assigned to any data and adherence to all pertinent federal and state regulations concerning the protection of the rights and welfare of all subjects were honored.
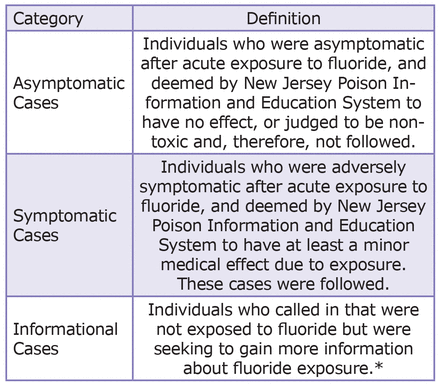
Approximately 210,000 total poison-related phone call records were searched for a subset of inclusion criteria cases, with 2,476 records meeting inclusion criteria. Information on the following parameters was obtained: age, gender, location of exposure, relationship of caller to patient, reason for exposure, type of fluoride-containing dentifrices involved in acute exposure, and medical outcomes of exposures. Data was then categorized by medical outcomes (Figure 2).
The medical outcome categories were defined by the parameters used by the AAPCC 2011 report. Those who were deemed to be “asymptomatic” did not develop any signs or symptoms as a result of the exposure. Individuals who were deemed to be “symptomatic” showed minor, moderate or major medical effects. “Minor effect” is defined as the patient developing some signs or symptoms as a result of the exposure, but they were minimally bothersome and resolved rapidly with no residual disability. “Moderate effect” is defined as the patient exhibiting signs or symptoms as a result of the exposure that were more pronounced or more prolonged. Usually, some form of treatment is indicated. Symptoms were not life-threatening and the patient had no residual disability. “Major effect” is defined as the patient exhibiting signs and symptoms as a result of the exposure that were life-threatening or resulted in residual disability or disfigurement. “Death” was defined as a patient dying as result of the exposure or as a direct complication of the exposure.10
Additionally, each case report was searched and reviewed individually to obtain specific information on circumstances of each case and the specific advice that was provided to the caller. Data analysis and incidence rates were calculated in Microsoft Excel. Graphs used 95% confidence intervals to calculate the significance in differences between groups.
Results
Frequency and Incidence of Acute Fluoride Exposure
Based on the inclusion criteria, the acute fluoride exposures reported were from pure fluoride (which included professionally applied and/or prescribed supplements), toothpaste with fluoride, mouth rinse with fluoride and multivitamin with fluoride (with and/or without iron).
“Pure fluoride” included gel forms of acidulated phosphate fluoride (APF) which contained 1.23% (12,300 ppm) fluoride, gel or foam of sodium fluoride (NaF) at 0.9% (9,040 ppm) fluoride and applied gel of sodium fluoride (NaF) at 0.5% (5,000 ppm) fluoride or stannous fluoride (SnF2) at 0.15% (1,000 ppm) fluoride. Overexposure/ingestion of NaF varnishes that were applied in-office by dental professionals were also included in the study, usually at 2.26% (22,600 ppm) fluoride preparation. Dietary fluoride supplements were in the form of tablets, lozenges or liquids. Most supplements contained sodium fluoride as the active ingredient with 1.0, 0.5 or 0.25 mg fluoride. The following highlights the conversion of fluoride to its ion/compound:
APF=1.23% F=2.7% NaF-
NaF=2% NaF=0.09% F-
SnF2=10% SnF2=2.5% F-
NaF Varnish=50 mg NaF-/ml=2.3% F-
Concentrations of fluoride in toothpaste ranged from 1,000 to 1,100 ppm. Fluoride in toothpaste came from 3 compounds (as permitted by the FDA): sodium monofluorophosphate (MFP), sodium fluoride (NaF) and stannous fluoride (SnF2). Product labels for 1,000 and 1,100 ppm products read as follows (note: 1,000 ppm equals 1.0 mg F/ml and 1,100 pm equals 1.1 mg F/ml):21
0.76% w/v MFP, which equals 1,000 ppm F (or 30 mg F/oz)
0.243% w/v NaF, which equals 1,100 ppm F (or 33 mg F/oz)
0.0454% w/v SnF2, which contains 1,100 ppm F (or 33 mg F/oz)
Classifications of Records, Based on Medical Outcome/Type
Toothpaste tube sizes varied; however, generally, a large tube of toothpaste was usually 6.4 oz and, therefore, contained 192 to 211 mg F. A small tube of toothpaste was usually 4.6 oz and contained 138 to 152 mg F. (6.4 oz tube (1,000 ppm F) × 30 mg F/oz=192 mg F).
Fluoride mouth rinse is a concentrated solution, and the most common fluoride compound used was sodium fluoride (0.05%, or 230 ppm fluoride). Multivitamins mostly contained sodium fluoride at 1.0, 0.5 or 0.25 mg fluoride.
As Table II depicts, there was a decreasing incidence of acute fluoride exposure over the years 2010 to 2012. Toothpaste with fluoride caused the highest incidence of calls related to acute fluoride exposure, each year and as a total.
Population Characteristics and Circumstances of Acute Fluoride Exposure Cases
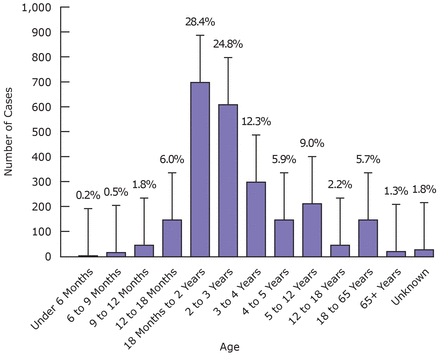
Age trends toward a unimodal distribution (Figure 3) among victims of acute fluoride exposure, with 53.2% of cases involving individuals between 18 months and 3 years of age. At a 95% confidence interval, the developmental age groups 18 months to 2 years and 2 to 3 years do not have overlapping bars (Figure 3), indicating a significant difference from other age groups. Of acute fluoride exposure victims, 79.9% were 5 years and under.
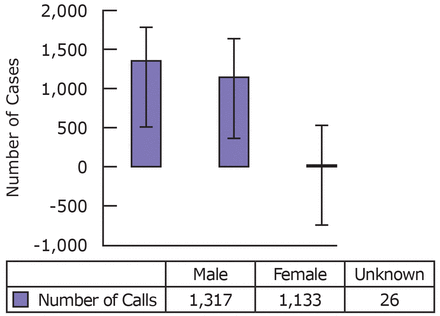
Males had a slightly higher incidence of reported acute fluoride exposures (Table III). Overlapping error bars at 95% confidence interval in Figure 4, however, show that this difference may not be significant (i.e. it may be due to chance). The majority of acute fluoride exposures occurred in one's own residence, while under the watch of the mother (Table III). Acute fluoride exposure was mainly unintentional.
Incidence of Phone Call Records Related To Acute Fluoride Exposure in New Jersey, By Year and Fluoride-Containing Product
Age Distribution of Acute Fluoride Exposure Cases in New Jersey, 2010 to 2012*
*Age increments on vertical axis broken down by cognitive development stages
Characteristics of Cases by Medical Outcome
As Table IV depicts, most cases would be considered asymptomatic. Of the symptomatic cases, there was mainly a minor medical effect and a small number had a moderate effect. There were no major medical effects or death, as deemed by the SPI (Table V). As Table VI demonstrates, most symptomatic cases were caused by toothpaste with fluoride. People had the most questions (informational cases) about pure fluoride.
Discussion
This study showed a decreasing trend of calls reporting acute fluoride exposure over the years 2010 to 2012 and follows the trend of decreasing calls since 2000 as per the 2011 AAPCC NPDS report. This decline may reflect the decreasing use of PCC's for acute exposures, possibly due to the increasing use of text over voice communication, and increased use and reliance on the internet.10 Additionally, the AAPCC report demonstrates that toothpaste comprised most of the fluoride-related calls. This is in accordance with this study's data, which demonstrated that toothpaste caused the highest incidence of acute fluoride exposure in New Jersey. Toothpaste is one of the most common at-home dental products containing fluoride and easily accessible. Children younger than 5 years tend to swallow toothpaste while brushing.19 Children 6 years and younger have a swallowing reflex that is not always well controlled.22
Characteristic and Circumstances of Acute Fluoride Exposures in New Jersey, 2010 to 2012
The age groups most affected by acute fluoride exposure (for both symptomatic and asymptomatic patients), in 2010 to 2012, was 18 months to 3 years of age. This is a vulnerable population consisting of toddlers in an inquisitive and exploratory sensorimotor stage. Other studies also demonstrate that the incidence of poisoning peaks between 1 and 3 years of age.14 Males and females seemed to be equally affected, although Swierzewski's study shows that males tend to be more affected.14 The most common site of acute fluoride exposure occurred in the home while children were under the watch of parents/guardians.
The most common reason for excess exposure was found to be unintentional/accidental. Common reasons cited for reasons of excessive or inappropriate ingestion were related to taking older siblings prescription, playing with products and accidentally ingesting, and accidentally ingesting more than prescribed, either by fault of guardian or individually. However, as many dental products at home do not taste good to children, several phone records cite that the child stopped ingesting the product on their own. Other phone records, however, cite that the children wanted to ingest the products due to their “bubble gum” and “orange” flavors which are common among pediatric dental products.
Gender Distribution of Acute Fluoride Exposure Cases in New Jersey, 2010 to 2012
Based on the results of this study, there were no life-threatening events or fatalities due to acute fluoride exposures, even when taken in doses higher than recommended or prescribed. Fluorides in available over-the-counter and prescription products are relatively safe and common acute doses have generally nontoxic and minor outcomes. It would require a very large amount of ingestion of fluoride-containing product to even require medical intervention, let alone direct fatality. Recall that it would take 67 mg of ingestion of fluoride for an average 2 year old child at 30 lbs. To put it into perspective, it would take nearly an entire tube of an average sized children's toothpaste tube to reach the acute toxic dose and ingestion of 3 tubes of toothpaste to reach the acute lethal dose.
Classification of Phone Call Records, Based on Medical Outcome
Symptomatic Cases, Categorized by Types of Symptoms
There was a small group (4.24% of total cases) of symptomatic cases, who exhibited minor and moderate effects of acute fluoride exposure. Most of these cases reported gastrointestinal symptoms (including nausea and vomiting and less frequently, diarrhea, abdominal pain and colored urine). The mechanism of toxicity is thought to occur by corrosive action, where fluoride reacts with hydrochloric acids in the stomach, resulting in gastrointestinal irritation.15 These symptoms were generally easily remedied with calcium as the antidote, in the form of milk, cheese, yogurt, etc., to bind the fluoride. Induced vomiting was not recommended by SPI's. The main concern was not poisoning, but rather aspiration or dehydration from the vomiting and the rare allergy. Based on this study, there were no hospitalizations necessary due to acute fluoride exposure.
New Jersey Poison Information and Education System, like other poison control centers across the nation, receives a large volume of fluoride-related calls largely concerning young children's excess exposure. While the warning labels are effective in alarming people to the dangers of excessive fluoride intake, this study found several cases of parents rushing their children to the hospitals due to the statement to “seek medical help right away.” It was found that it was unnecessary to do so; all of the children were discharged and did not need further treatment (as confirmed by a follow-up call from New Jersey Poison Information and Education System). Visits to the emergency department can cost resources and it may be more cost-effective for the label to indicate making a phone call to a PCC first. The authors support ADA's statement that the FDA warning labels may be making parents and guardians overly frightened.
Breakdown of Medical Outcome Classification by Product Type
This study is important for the dental hygienist in light of clinical practice, patient education and the current controversies in the media regarding fluoride.
Clinical Practice
Guidelines for in-office ingestion of fluoride: If the child patient in the dental chair accidentally swallows fluoride during an in-office fluoride treatment, the child should be given water and any calcium-containing product (milk, cheese, yogurt, ice cream) as soon as possible. If vomiting occurs and does not stop, and/or severe abdominal pain, it may be necessary to take the child to the emergency department. The main concern with vomiting is dehydration. If the child is vomiting, make sure they are seating up right and not sleeping on their back to prevent aspiration.
When prescribing/recommending fluoride as a supplement: As dental professionals, it is important to perform a caries risk assessment before making recommendations associated with preventing or controlling caries. As the American Academy of Pediatric Dentistry (AAPD) recommends, dental caries risk assessment should be based on a patient's age, biological factors, protective factors and clinical findings.25 Biological factors include primary caregivers having active caries, low socioeconomic status, the number of meal sugar-containing snacks or beverages consumed per day, the patient having special health care needs, and/or the patient is a recent immigrant. Protective factors include whether the patient receives optimally-fluoridated drinking water, other fluoride supplements and the patient follows regular dental home care and in-office visits. Clinical findings include having more than 1 decayed/missing/filled surfaces, having active white spot lesions or enamel defects, elevated mutans streptococci levels, or plaque on teeth.25
It is critical to assess a child's total fluoride exposure from all sources (food, drink, optimally treated water, toothpaste, supplements, topical applications in-office, etc.) when developing oral care recommendations and treatment plans.23 The ADA, AAPD and the American Academy of Pediatrics (AAP) encourages practitioners to calculate appropriate dose based on a child's total fluoride exposure and caries risk status.17,21,25 Fluoride supplements are recommended only for children living in non-fluoridated areas and at high risk for tooth decay.23 While studies demonstrate that fluoride can provide a tremendous benefit,2-5 and this study supports the relative safety of fluoride, a risk still remains with overexposure calling for its judicious application.4,6-8 Fluoride therapy can be customized, and it must be remembered that modifications to therapy are necessary based on a patient's changing risk assessment, disease status and fluoride exposure.
If fluoride levels in water are unknown, drinking water should be tested for fluoride content before supplements are prescribed. If the water comes from a public or community water supply, the local water supplier can help to determine the amount of fluoride. The Environmental Protection Agency (EPA) regulates fluoride in drinking water, although the decision to fluoridate a water supply at all is made by the state or local municipality.24 The CDC and EPA's websites can be valuable resources to determine water fluoridation levels. If the water source is a private well, it will need to be tested and the results obtained from a certified laboratory.24
If a fluoride prescription is deemed necessary, it should be written legibly and distinguish between mg F and mg NaF. No more than 120 mg of fluoride in a bottle should be prescribed to avoid possible lethal dose, although multiple refills are permitted.
Dental hygienists must remember to carefully evaluate new fluoride products in the market, and review laboratory and clinical evidence supporting the efficacy of these products before applying them in-office or recommending them to patients.
Education
Dental hygienists provide valuable information to their patients regarding home care and effective dental products. It is important that they remember to remind patients of proper dosage and safety measures when handling these products at every visit. The act of reminding helps to solidify knowledge and good habits. Toothpaste is the number one fluoride-containing dentifrice in acute fluoride exposures; therefore, dental hygienists should educate and remind parents to put away their toothpaste in a place at home that is far from reach from their toddlers.
A few days after birth and even before the teeth erupt, caregivers should clean their child's mouth and gums with a soft moistened washcloth or gauze pad at bath time. This helps ready the child for the toothbrush cleaning to come, and they become accustomed to having something in their mouth in such a manner.26 Additionally, this routine will wash off bacteria that could otherwise damage the infant teeth as they come in.27 For children younger than 3 years of age, caregivers should start brushing children's teeth as soon as they begin to come into the mouth with fluoridated toothpaste – no more than a smear or size of a grain of rice.17 For children 3 to 6 years of age, caregivers should dispense no more than a pea-sized amount of fluoridated toothpaste.17 Children should always be supervised to ensure that they use the appropriate amount of toothpaste and to minimize swallowing of toothpaste. It is important to provide counseling to these caregivers at every dental visit with the use of clear description, visual aids and demonstration to ensure that the appropriate amount of toothpaste is used. Studies show that caregivers apply up to twice the recommended amount of toothpaste – it is imperative that they are well-educated.18,20
As part of oral health education, dental hygienists can also assure concerned parents that if too much fluoride intake is suspected, it is helpful to have their child ingest a calcium-containing product. It is not recommended to induce vomiting. If any uncertainties arise, patients should be educated on the role of the poison control center which is open 24 hours a day and 365 days a year.
This study revealed that many informational calls were made by concerned parents and guardians regarding the safety of fluoride, further illustrating the current controversies. Recently, many dental hygienists are faced with questions from patients and parents of patients regarding the safety of fluoride. While too much of any substance can be harmful, patients can be assured that the benefits of fluoride in fighting tooth decay outweigh potential harms. If patients express doubts about fluoride use and evidence-based discussions do not placate concerns, dental professionals must respect the patient's position and emphasize the need for proper nutrition and meticulous oral hygiene. Dietary counseling and education on sugar, forms of sugar and unhealthy vs. healthy snacks is important.
When educating patients and/or caregivers, it is imperative that dental hygienists are conscious of their communication techniques to help drive motivation and compliance. The patient will be most motivated to learn when good rapport, speech, tone of voice, body language and facial expression have been established.28 Basic principles of teaching have been shown to increase the effectiveness of compliance, including:28
Presenting small amounts of information at one time in simplified words
Letting the patient set their own pace by making sure they have learned the technique before moving on to teach other things
Supervising the patient and making sure they are practicing the correct technique
Providing feedback during visits and teaching the patient self-evaluation tools
Using positive reinforcement
Taking the time to perform the correct assessment and employing proper communicative techniques during education are the fundamentals of successful compliance.
There were some limitations to the study due to its retrospective nature. The data is limited in that it only deals with cases reported to New Jersey Poison Information and Education System. That is, the actual number of actual exposures that occur in the population is unknown, as they may go unreported. Additionally, the data is all based on history given, and some were estimates. There was some missing and unknown data in some subcategories, including age, gender, locational site, medical outcome and reason for exposure. It is possible that some of the adverse reactions to ingestion of the products were related to ingredients other than fluoride.
Future studies may want to test and verify accurate amounts of fluoride ingested, rather than accepting caregiver reports. In the future, it would be helpful to separate and evaluate dental products based on the type of fluoride present (whether sodium fluoride, stannous fluoride, etc.) instead of grouping all toothpaste, mouth rinse, pure fluoride and multivitamins with fluoride together. It was difficult to separate the types of fluoride in this study due to the second-hand nature of obtaining the data based on personal reports.
Conclusion
Based on the results in the study, there was no incidence of lethality or toxicity due to acute fluoride exposure in New Jersey from 2010 through 2012. Almost all cases had no medical outcomes; very few cases had mostly minor symptoms from acute fluoride exposure. The benefits of fluoride generally outweigh the risks.
Dental hygienists are advised to perform caries risk assessment and evaluate overall fluoride exposure for each patient before making recommendations associated with preventing or controlling caries. Dental hygienists should remind patients or caregivers to call the American Association of Poison Control Center (800-222-1222) immediately if fluoride toxicity is suspected.
Acknowledgments
The authors would like to thank Dr. Steven Marcus, Dr. Teri Lassiter, and Dr. William Halperin of Rutgers University for their help with this research study.
Footnotes
Sneha Shah, RDH, MPH, is employed in private practice and in extended sales at SolutionReach Patient Relationship Management Company. Samuel Quek, DMD, MPH, is a Professor, Director of General Practice Residency Program, Director of Division of Hospital Dentistry, at the School of Dental Medicine at Rutgers Department of Diagnostic Sciences. Bruce Ruck, PharmD, is Diplomate of the American Board of Applied Toxicology (DABAT) at New Jersey Poison Information and Education System.
This study supports the NDHRA priority area, Occupational Health and Safety: Investigate methods to decrease errors, risks and or hazards in health care and their harmful impact on patients.
Disclosure
Toxicall is a registered trademark of Computer Automatic Systems, Inc.
- Copyright © 2016 The American Dental Hygienists’ Association