Abstract
Aims/hypothesis
Diabetes prevention and care are limited by lack of screening. We hypothesised that screening could be done with a strategy similar to that used near-universally for gestational diabetes, i.e. a 50 g oral glucose challenge test (GCT) performed at any time of day, regardless of meal status, with one 1 h sample.
Methods
At a first visit, participants had random plasma and capillary glucose measured, followed by the GCT with plasma and capillary glucose (GCTplasma and GCTcap, respectively). At a second visit, participants had HbA1c measured and a diagnostic 75 g OGTT.
Results
The 1,573 participants had mean age of 48 years, BMI 30.3 kg/m2 and 58% were women and 58% were black. Diabetes (defined by WHO) was present in 4.6% and prediabetes (defined as impaired glucose tolerance [2 h glucose 7.8–11.1 (140–199 mg/dl) with fasting glucose ≤6.9 (125 mg/dl)] and/or impaired fasting glucose with plasma glucose 6.1–6.9 mmol/l [110–125 mg/dl]) in 18.7%. The GCTplasma provided areas under the receiver-operating-characteristic curves of 0.90, 0.82 and 0.79 for detection of diabetes, diabetes or prediabetes, and prediabetes, respectively, all of which were higher than GCTcap, random and capillary glucose, and HbA1c (p < 0.02 for all). The performance of GCTplasma was unaffected by time after meals or time of day, and was better in blacks than whites, but otherwise comparable in men and women, and in groups with differing prevalence of glucose intolerance. GCTplasma screening would cost approximately US$84 to identify one person with previously unrecognised diabetes or prediabetes.
Conclusions/interpretation
GCT screening for prediabetes and previously unrecognised diabetes would be accurate, convenient and inexpensive. Widespread use of GCT screening could help improve disease management by permitting early initiation of therapy aimed at preventing or delaying the development of diabetes and its complications.
Similar content being viewed by others
Introduction
Many diabetes patients have poor control of glucose and associated cardiovascular disease (CVD) risk factors [1, 2]. While the causes of inadequate management are complex [3, 4], the problem is due in part to recognition relatively late in the natural history of glucose intolerance [5]. As beta cell function and mass are lost [6, 7], it becomes progressively more difficult to lower glucose levels to normal [8]. In contrast, detection earlier in the natural history at the stage of prediabetes can enable lifestyle change and/or use of pharmacological agents to keep glucose levels near-normal, prevent or delay the development of diabetes, and improve other CVD risk factors [9, 10]. However, the 20% of Americans with prediabetes [11] and 6 million Americans with diabetes [12] are undiagnosed because most Americans are not screened in any systematic way [13].
The most sensitive way to screen is to use OGTTs, but these are inconvenient [14]. Since early glucose intolerance is often pathophysiologically similar to gestational diabetes (high glucose levels after a challenge), it seemed possible that screening could be done using a strategy similar to that used for gestational diabetes, i.e. an oral glucose challenge test (GCT), in which 50 g glucose are given at any time of day, without a prior fast, and glucose levels are measured 1 h later. If the glucose levels exceed a cut-off, patients then have an OGTT. We hypothesised that such a strategy could constitute an effective, convenient, low-cost method of screening in non-pregnant adults.
Methods
Participants
Between 1 January 2005 and 31 March 2008 participants aged 18 to 87 years took part in this Screening for Impaired Glucose Tolerance Study (SIGT). Volunteer participants were recruited with posters, flyers, presentations and notices in the media, and included employees of the Grady Health System, Emory HealthCare, and Emory University and Morehouse Schools of Medicine, as well as members of the community. Individuals were eligible if they had no prior diagnosis of diabetes, were not pregnant or nursing, not taking glucocorticoids and were well enough to have worked during the previous week (without requiring actual employment). During recruitment, 4,024 individuals expressed initial interest in the study, 2,111 were scheduled for first visits (selected largely on the basis of need to balance participant sex and race), 1,658 completed first visits, 1,581 completed the protocol and 1,573 had complete GCT and OGTT data (see Electronic supplementary material [ESM] Fig. 1).
Protocol
The study was approved by the Emory University Institutional Review Board and was performed in outpatients in the general clinical research centres at Emory University and Grady Memorial Hospitals. At the first visit, which did not require a prior fast and was scheduled during the work day, participants had random plasma glucose (RPG) and random capillary glucose (RCG) measured (Freestyle; Abbott, North Chicago, IL, USA). Participants then drank 50 g oral glucose (Trutol; NERL Diagnostics, East Providence, RI, USA) within 5 min, with measurement of plasma and capillary glucose after 1 h (GCTplasma and GCTcap, respectively). To avoid confounding via change in behaviour, participants were not given these results. At a second visit (average of 13 days after first visit), a 75 g OGTT was begun before 11.00 hours following an overnight fast, with samples at baseline, 1 and 2 h; blood was also obtained for measurement of plasma lipids and HbA1c.
Measurements
Glucose samples were obtained using sodium fluoride/oxalate preservative and plasma frozen at −80°C within 30 min. Chemical analyses were performed in the central clinical laboratory of the Grady Health System using an LX-20 device (Beckman-Coulter, Brea, CA, USA). HbA1c was measured with an assay approved by the National Glycohemoglobin Standardization Program (Beckman Synchron, Fullerton, CA, USA). Sex-adjusted values for HDL-cholesterol (and waist circumference) were expressed relative to cut-offs for the metabolic syndrome defined by National Cholesterol Education Program (NCEP) Adult Treatment Panel III criteria.
Estimated costs for screening
Projection of costs for screening with GCTplasma assumed follow-up testing with an OGTT if the screening test exceeded a 70% specificity cut-off. We used current Medicare reimbursements of US$6.64 for a gestational diabetes GCT (including 50 g glucose) and $17.99 for the OGTT, assumed 5 min of medical assistant time would be needed ($1.13) for administration of the glucose and expressed costs per case identified, cases being defined as (1) diabetes; (2) prediabetes110 (see below).
Analysis
Of the 1,581 participants, eight (0.5%) did not have 1 h GCT plasma values; data of the remaining 1,573 participants were used in the primary analyses. Of the 1,573, one did not have an RCG value and two had no triacylglycerol values. Since for analyses involving RCG and triacylglycerol, the number of participants was reduced by less than one-half per cent, no methods of adjustment or imputation of missing data were employed.
Normal glucose tolerance was defined by American Diabetes Association (ADA) criteria (fasting glucose <5.6 mmol/l [100 mg/dl], 2 h glucose <7.8 mmol/l [140 mg/dl]). Impaired fasting glucose (IFG) was defined as IFG with plasma glucose 5.6–6.1 (100–109 mg/dl) (IFG100) and IFG with plasma glucose 6.1–6.9 (110–125 mg/dl) (IFG110), respectively, both with 2 h glucose <7.8 (140 mg/dl). IGT was defined as 2 h glucose 7.8–11.1 (140–199 mg/dl) with fasting glucose ≤6.9 (125 mg/dl). Diabetes was defined as fasting glucose ≥7.0 mmol/l (126 mg/dl) or 2 h glucose ≥11.1 mmol/l (200 mg/dl). We focused particularly on IGT or IFG110 (prediabetes110), and on diabetes, IGT or IFG110 (dysglycaemia110), since these categories are consistent with the WHO definition of prediabetes [15] and such glucose levels confer increased risk of CVD and mortality [16–18]. The prevalence of glucose intolerance in the 1,196 study participants over the age of 40 years in the study population was compared with that in age-matched participants in the National Health and Nutrition Examination Survey III (NHANES III) population (www.cdc.gov/nchs/products/elec_prods/subject/nhanes3.htm, accessed 15 May 2009), after restricting the latter to black and white participants without previously known diabetes and with OGTTs performed at least 8 h after a meal and before 11.00 hours, with OGTT glucose samples obtained within 115 to 125 min after baseline.
The discriminative effectiveness of screening was evaluated by the area under receiver-operating-characteristic curves (AROCs). Bootstrap methodology was applied to validate the predictive ability of 1 h GCTplasma (bootstrapping involves drawing a large number of random samples, with replacement and with all bootstrapped samples the same size as the original sample; this methodology provides a sampling distribution of the AROC from which validation statistics can be calculated.) In this application, 500 bootstrap replicates were drawn to calculate the over-optimism of sample-calculated AROCs, calibration slopes (a measure of generalisability to other populations) and maximum absolute error in AROCs. Methods were applied to prediction of diabetes, dysglycaemia110 and prediabetes110. AROCs with different screening tests were compared with the method of DeLong et al. [19]. To assess the performance of GCTplasma in subgroups with different pretest probability of glucose intolerance, we examined subgroups with differences in risk factors such as age. Statistical analyses were conducted using S-Plus, version 7 (Insightful, Seattle, WA, USA) and Stata, version 10 (Stata, College Station, TX, USA).
Results
The 1,573 study participants had an average age of 48 years and BMI 30.3 kg/m2, and 58% were women and 58% black. Of the 1,573, 4.6% had diabetes, 18.7% prediabetes110 and 23.3% dysglycaemia110. In the 1,178 study participants aged 40 to 74 years, the proportions with previously unrecognised diabetes and prediabetes110 (5.3% and 21.8%) were no greater than those in the 1,807 age-matched participants in the NHANES III population (11.0% and 27.6%, p < 0.0001 for both). Extrapolating the study proportions to the US population, approximately 40 million Americans would be expected to have unrecognised diabetes or prediabetes110. As shown in Table 1, those with abnormal glucose tolerance were older and heavier; those with IFG only were less likely to be black or women; and those with IGT only were more likely to be women. Although HbA1c levels above the normal range were uncommon except in participants with diabetes (6.9% of participants tested above 6.0%), many of those with diabetes or prediabetes110 were at increased risk of CVD because of systolic blood pressure above 130 mmHg (36.9%) or LDL-cholesterol above 5.6 mmol/l (100 mg/dl; 69.4%).
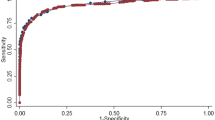
GCTplasma was a strong indicator of unrecognised glucose intolerance (Fig. 1). Thus the AROCs were: 0.79 (95% CI 0.76–0.82) to identify prediabetes110; 0.82 (95% CI 0.79–0.84) for dysglycaemia110; and 0.90 (95% CI 0.86–0.93) for diabetes (p < 0.001 for all). Bias-corrected AROCs differed only in the third or fourth decimal place. Calibration slopes for all categories of glucose intolerance were greater than 0.99, indicating excellent generalisability to other populations. Maximum absolute errors (bias) for all AROCs were 0.001 or less. GCTplasma identified IGT (AROC 0.80; 95% CI, 0.77–0.82) better than IFG110 (AROC 0.72; 95% CI, 0.67–0.77). In comparisons at different times after meals and times of day (see ESM Table 1), the AROCs for GCTplasma to identify unrecognised diabetes, dysglycaemia110 and prediabetes110 were unaffected by postprandial time or time of day (p > 0.15 for all).
ROC curves showing performance of GCTplasma in identifying (a) prediabetes110 (any IGT or IFG110), (b) dysglycaemia110 (diabetes or prediabetes110) and (c) diabetes. AROC: (a) 0.79 (95% CI 0.76–0.72); (b) 0.82 (95% CI 0.79–0.84); (c) 0.90 (95% CI 0.86–0.93). The values along the curves show different glucose cut-off levels for 50 g glucose challenge test (any time of day, no fast needed) in mmol/l with values in mg/dl in parentheses. The dashed line shows screening test performance equivalent to chance (AUC 0.5)
The performance of GCTplasma as a screen in subgroups with higher or lower pretest probability of having diabetes, dysglycaemia110 or prediabetes110 is shown in Table 2. The likelihood of glucose intolerance was significantly increased by well-accepted risk factors for glucose intolerance. Each factor also generally contributed to the probability of having GCTplasma ≥7.8 mmol/l (140 mg/dl), but had little impact on the AROCs for GCTplasma except that AROCs were higher for blacks than whites (p < 0.05 for all) and AROCs for identification of dysglycaemia110 or prediabetes110 in participants with triacylglycerol <1.69 mmol/l (150 mg/dl) were higher than in those with triacylglycerol ≥1.69 (150) (p < 0.05 for both). There were also no general trends with respect to markers of insulin resistance as components of the metabolic syndrome. Thus, the presence of diabetes risk factors was associated with a higher prevalence of glucose intolerance and would increase the positive predictive value of GCTplasma as a screening test, but had little impact on the ability of GCTplasma to detect glucose intolerance.
AROC analysis was also used to compare GCTplasma with other tests that would also be convenient for screening, e.g. RPG, GCTcap, RCG and HbA1c (Table 3). When glucose was assessed with challenge vs random tests, the challenge tests were generally superior for GCTplasma compared with RPG and GCTcap vs RCG (p < 0.05 for all); GCTplasma was also superior to GCTcap (p < 0.001 for all) and HbA1c (p < 0.001 for prediabetes110 and dysglycaemia110; p = 0.018 for diabetes). We also compared FPG to plasma glucose measured at 1 h during the OGTT, with both tests obtained after similar dietary preparation and 1 h apart. The OGTT 1 h plasma glucose was superior to FPG for detecting dysglycaemia110 and prediabetes110 (p < 0.001 for both).
Table 4 shows the effect of different GCTplasma and GCTcap cut-offs on sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). The lower cut-offs provide greater sensitivity and NPV, but reduced PPV, since specificity is lower and PPV responds to the underlying presence of the abnormalities in the population. A relatively high-specificity GCTplasma cut-off of 7.8 mmol/l (140 mg/dl) provided good specificity, sensitivity and NPV, with acceptable PPV. Use of a lower cut-off would increase the sensitivity, but a lower percentage of those with a positive test would prove to have glucose intolerance. Conversely, screening with higher cut-offs would provide greater likelihood of finding glucose abnormalities among those who screened positive (higher PPV), at the cost of reduced sensitivity and NPV. Higher specificity cut-offs also tend to be more cost-effective [20]. GCTcap cut-offs tended to be about 1.1 mmol/l (20 mg/dl) higher than the comparable GCTplasma cut-offs, with somewhat lower sensitivity and specificity.
Finally, we projected costs for GCTplasma screening with the 7.8 mmol/l (140 mg/dl) cut-off. Of our 1,573 participants, 622 (40%) would have exceeded the cut-off and had a follow-up OGTT, of whom 280 (45%) would have had glucose intolerance (66 diabetes, 214 prediabetes110). Initial screening would incur Medicare costs of $7.77 per participant = $12,222; follow-up OGTTs would incur Medicare costs of $17.99 per positive screen = $11,190. Total costs would therefore be $23,412 or $84 per case identified.
Discussion
Principal findings
The infrequency of screening for early glucose intolerance in non-pregnant individuals [13] may be due in part to lack of a screening strategy suitable for the general population. We demonstrate that a screening approach modelled on that for gestational diabetes could be such a strategy. Screening with GCTplasma provided AROC 0.79, 0.82 and 0.90 to detect prediabetes110, dysglycaemia110 and previously unrecognised diabetes respectively. Screening performance was generally consistent across different times after meals and different times of day, as well as in subgroups with higher and lower pretest probability of glucose intolerance. GCTplasma screening appears to be accurate, convenient and widely applicable, and the test would be relatively inexpensive in populations such as ours ($84 per case of diabetes or prediabetes identified).
Comparisons with other studies
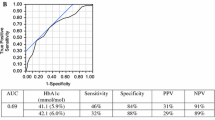
Previous studies of screening have focused on risk factor questionnaires, glucose-related measures and risk factor combinations of demographics, anthropometrics and laboratory measurements. Questionnaires are inexpensive and some have been reported to provide AROC for diabetes of ~0.80 [21], comparable to that of RPG in the present study, with most providing AROC 0.70–0.75 [22]. In our population, the Diabetes Risk Calculator [23] provided AROC 0.70 for diabetes and 0.67 for prediabetes110. Such inefficiency has led to greater interest in glycaemic indicators such as HbA1c [24], which identified retinopathy almost as well as fasting or 2 h post-challenge glucose in a Pima Indian population [25], but in other populations performed less well than fasting glucose in identifying diabetes [26] and was very insensitive in detecting IGT [27], comparable to findings in our study. Problems in use of HbA1c probably reflect large inter-individual variability [28].
Use of glucose levels for screening has revealed that post-challenge glucose is a more uniform predictor of progression from IGT to diabetes as compared with fasting glucose [29]. Moreover, fasting glucose measurements often fail to recognise both IGT and diabetes [30, 31], and poor sensitivity is obtained even with lower cut-off levels [32, 33]. Previous studies of RPG and RCG have frequently not included AROC values [34, 35], but RCG can be efficient when combined in an equation with postprandial time, age, sex and BMI [36]. Such an equation provided AROC comparable to that of GCTplasma in the present study, but busy practitioners might find the equation inconvenient.
There has also been interest in screening with risk factor-based predictive models such as the metabolic syndrome. However, the AROCs for predictive models have generally been lower than those for GCTplasma in the present study; indeed, in the San Antonio Heart Study, the best model to predict incident diabetes was no better than the 1 h OGTT glucose value alone [37, 38], a measurement similar to GCTplasma. Moreover, it might be easier to implement GCT screening than risk factor-based screening in real-world practice. Risk factor guidelines would require an extra visit for fasting morning samples (glucose, lipids), which patients might be reluctant to schedule [14]. Risk factor-based screening demands consistent methodology, but lipid values vary considerably (data from the College of American Pathologists show a coefficient of variation of 8–16% for HDL vs 2–3% for glucose [39]). Risk factor-based screening may be poorly applicable to multi-ethnic populations [40] and it might also be difficult for providers to tell whether risk factor-based evaluation has been conducted. In contrast, GCT screening lacks such limitations: patients need not be fasting, the test can be done at any time of day, glucose measurements are highly consistent and the process of screening is readily identifiable.
Policy recommendations for screening must be driven by evidence of clinical benefit, predictive utility and reasonable cost. In the Diabetes Prevention Program, the direct medical costs of identifying one participant with IGT (RCG screening followed by OGTT) were US$139 [41]. The Center for Disease Control group projected the minimum cost of identifying a case of diabetes or prediabetes as $176 (comparing RCG, HbA1c, FPG and OGTT) [42] or $172 (comparing RCG, FPG and HbA1c) [35]. However, GCTplasma screening followed, if positive, by an OGTT would incur direct costs of only $84 per case identified; the GCTplasma approach is both more accurate and less expensive.
Limitations
Our study has limitations. Having studied blacks and whites, we cannot be sure that the findings will apply to other racial or ethnic groups. We also had few tests that were initiated after 15.00 hours. Since glucose tolerance is better later in the day [43], it is possible that a GCTplasma in the late afternoon might be falsely negative in predicting results from OGTTs performed in the morning, but our conditions do reflect the situation most likely to be convenient for patients and providers. To limit possible confounding by variation in day-to-day metabolic status, we also excluded participants with illness severe enough to make them miss work, since GCTplasma accuracy could be reduced if participants were screened when ill (possibly more insulin-resistant) and follow-up OGTTs performed when they were well again. We did not measure intra-participant variability of the GCT, but such variability, along with that of the OGTT, should be accounted for in the AROC analyses. We also, to date, have not validated our findings in a separate population, and so generalisability of our results might be reduced because our participants were self-selected. However, the prevalence of unrecognised diabetes and prediabetes110 in our study was no higher than that in NHANES III, GCTplasma AROCs were generally unaffected by the presence or absence of diabetes risk factors and bootstrapping analyses showed little bias.
Implications
Americans have a high lifetime risk of diabetes [44]; many patients already have early diabetes complications and abnormal CVD risk factors at first presentation [45, 46]; risk factor management can decrease CVD [47]; progression from prediabetes to diabetes can be prevented or delayed [9, 10]; and screening for prediabetes should be cost-effective [48]. Although such findings contributed to the call by NIDDK/ADA to screen for prediabetes [49], screening is infrequent [13], due in part to the lack of a suitable screening test. Use of the GCT could solve this problem. It should be noted that because of the importance of identifying gestational diabetes at the first opportunity, GCT screening during pregnancy uses high sensitivity cut-offs. In contrast, GCT screening of non-pregnant individuals can use cut-offs that provide higher specificity (and greater cost-effectiveness [20, 34]), since patients missed at an initial screen would probably be detected on repeat screening 2 to 3 years later, which would still be early in their natural histories.
Conclusions
Lack of a good strategy to identify early glucose intolerance, possibly 10 years or more prior to current identification of diabetes, is a major impediment to better care. GCT screening would be an accurate, convenient and relatively inexpensive way to find prediabetes and previously unrecognised diabetes, providing a major opportunity to improve the health of the ~40 million Americans who have these problems. Moreover, recognition early in the patient’s natural history would allow the use of therapies to slow the progression of glucose intolerance and the development of associated microvascular and macrovascular complications.
Abbreviations
- ADA:
-
American Diabetes Association
- AROC:
-
Area under the receiver-operating-characteristic curve
- CVD:
-
Cardiovascular disease
- Dysglycaemia110 :
-
Type 2 diabetes or IGT or IFG110
- FPG:
-
Fasting plasma glucose
- GCT:
-
Glucose challenge test
- GCTcap:
-
Glucose challenge test: capillary
- GCTplasma:
-
Glucose challenge test: plasma
- IFG:
-
Impaired fasting glucose
- IFG100 :
-
Impaired fasting glucose with plasma glucose 5.6–6.1 mmol/l (100–109 mg/dl)
- IFG110 :
-
Impaired fasting glucose with plasma glucose 6.1–6.9 mmol/l (110–125 mg/dl)
- IGT:
-
Impaired glucose tolerance
- NCEP:
-
National Cholesterol Education Program
- NHANES-III:
-
National Health and Nutrition Examination Survey III
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- Prediabetes110 :
-
IGT or IFG110
- RCG:
-
Random capillary glucose
- RPG:
-
Random plasma glucose
References
Saaddine JB, Cadwell B, Gregg EW et al (2006) Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Ann Intern Med 144:465–474
Hoerger TJ, Segel JE, Gregg EW, Saaddine JB (2008) Is glycemic control improving in U.S. adults? Diabetes Care 31:81–86
Absetz P, Valve R, Oldenburg B et al (2007) Type 2 diabetes prevention in the “real world”: one-year results of the GOAL implementation trial. Diabetes Care 30:2465–2470
Phillips LS, Branch WT Jr, Cook CB et al (2001) Clinical inertia. Ann Int Med 135:825–834
Holman RR (1998) Assessing the potential for alpha-glucosidase inhibitors in prediabetic states. Diabetes Res Clin Pract 40(Suppl):S21–S25
Weyer C, Bogardus C, Mott DM, Pratley RE (1999) The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104:787–794
Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110
Nichols GA, Alexander CM, Girman CJ, Kamal-Bahl SJ, Brown JB (2006) Treatment escalation and rise in HbA1c following successful initial metformin therapy. Diabetes Care 29:504–509
Buchanan TA (2007) (How) can we prevent type 2 diabetes? Diabetes 56:1502–1507
Pi-Sunyer X, Blackburn G, Brancati FL et al (2007) Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 30:1374–1383
Cowie CC, Rust KF, Ford ES et al (2009) Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care 32:287–294
National Centre for Chronic Disease Prevention and Health Promotion (2007) 2007 National diabetes fact sheet. Available from www.cdc.gov/diabetes/pubs/factsheet07.htm, accessed 1 March 2008
Ealovega MW, Tabaei BP, Brandle M, Burke R, Herman WH (2004) Opportunistic screening for diabetes in routine clinical practice. Diabetes Care 27:9–12
Leiter LA, Barr A, Belanger A et al (2001) Diabetes screening in Canada (DIASCAN) study: prevalence of undiagnosed diabetes and glucose intolerance in family physician offices. Diabetes Care 24:1038–1043
Twigg SM, Kamp MC, Davis TM, Neylon EK, Flack JR (2007) Prediabetes: a position statement from the Australian Diabetes Society and Australian Diabetes Educators Association. Med J Aust 186:461–465
The DECODE Study Group on behalf of the European Diabetes Epidemiology Group (1999) Glucose tolerance and mortality: comparison of WHO and American Diabetic Association diagnostic criteria. Lancet 354:617–621
Meigs JB, Nathan DM, D'Agostino RB Jr, Wilson PWF (2002) Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care 25:1845–1850
Sorkin JD, Muller DC, Fleg JL, Andres R (2005) The relation of fasting and 2-h postchallenge plasma glucose concentrations to mortality: data from the Baltimore Longitudinal Study of Aging with a critical review of the literature. Diabetes Care 28:2626–2632
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
CDC Diabetes Cost-Effectiveness Study Group (1998) The cost-effectiveness of screening for type 2 diabetes. JAMA 280:1757–1763
Schulze MB, Hoffmann K, Boeing H et al (2007) An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 diabetes. Diabetes Care 30:510–515
Aekplakorn W, Bunnag P, Woodward M et al (2006) A risk score for predicting incident diabetes in the Thai population. Diabetes Care 29:1872–1877
Heikes KE, Eddy DM, Arondekar B, Schlessinger L (2008) Diabetes Risk Calculator: a simple tool for detecting undiagnosed diabetes and pre-diabetes. Diabetes Care 31:1040–1045
Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB (2008) A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab 93:2447–2453
McCance DR, Hanson RL, Charles M-A et al (1994) Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ 308:1323–1328
Rohlfing CL, Little RR, Wiedmeyer H-M et al (2000) Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care 23:187–191
Motala AA, Omar MAK (1992) The value of glycosylated haemoglobin as a substitute for the oral glucose tolerance test in the detection of impaired glucose tolerance (IGT). Diab Res Clin Prac 17:199–207
Kilpatrick E, Maylor PW, Keevil BG (1998) Biological variation of glycated hemoglobin: implications for diabetes screening and monitoring. Diabetes Care 21:261–264
Edelstein SL, Knowler WC, Bain RP et al (1997) Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 46:701–710
Mannucci E, Bardini G, Ognibene A, Rotella CM (1999) Comparison of ADA and WHO screening methods for diabetes mellitus in obese patients. Diabetic Med 16:579–585
Barzilay JI, Spiekerman CF, Wahl PW et al (1999) Cardiovascular disease in older adults with glucose disorders: comparison of American Diabetes Association criteria for diabetes mellitus with WHO criteria. Lancet 354:622–625
Chang C-J, Wu J-S, Lu F-H, Lee H-L, Yang Y-C, Wen M-J (1998) Fasting plasma glucose in screening for diabetes in the Taiwanese population. Diabetes Care 21:1856–1860
Schmidt MI, Duncan BB, Vigo A et al (2003) Detection of undiagnosed diabetes and other hyperglycemia states. Diabetes Care 26:1338–1343
Johnson SL, Tabaei BP, Herman WH (2005) The efficacy and cost of alternative strategies for systematic screening for type 2 diabetes in the U.S. population 45–74 years of age. Diabetes Care 28:307–311
Zhang P, Engelgau MM, Valdez R, Cadwell B, Benjamin SM, Narayan KMV (2005) Efficient cutoff points for three screening tests for detecting undiagnosed diabetes and pre-diabetes: an economic analysis. Diabetes Care 28:1321–1325
Hoerger TJ, Hicks KA, Sorensen SW et al (2007) Cost-effectiveness of screening for pre-diabetes among overweight and obese U.S. adults. Diabetes Care 30:2874–2879
Abdul-Ghani MA, Williams K, Defronzo RA, Stern M (2007) What is the best predictor of future type 2 diabetes? Diabetes Care 30:1544–1548
Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA (2008) One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care 31:1650–1655
Chemistry/Therapeutic Drug Monitoring Survey (2008) College of American Pathologists (Northfield, IL, USA). Available from www.cap.org, accessed 15 May 2009
Park Y-W, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB (2003) The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 163:427–436
Herman WH, Brandle M, Zhang P, The Diabetes Prevention Program Research Group et al (2003) Costs associated with the primary prevention of type 2 diabetes mellitus in the Diabetes Prevention Program. Diabetes Care 26:36–47
Zhang P, Engelgau MM, Valdez R, Benjamin SM, Cadwell B, Narayan KMV (2003) Costs of screening for pre-diabetes among U.S. adults. Diabetes Care 26:2536–2542
Troisi RJ, Cowie CC, Harris MI (2000) Diurnal variation in fasting plasma glucose: implications for diagnosis of diabetes in patients examined in the afternoon. JAMA 284:3157–3159
Narayan KMV, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF (2003) Lifetime risk for diabetes mellitus in the United States. JAMA 290:1884–1890
Thaler LM, El-Kebbi IM, Ziemer DC, Gallina DL, Dunbar VG, Phillips LS (1998) High prevalence of albuminuria among African-Americans with short duration of diabetes. Diabetes Care 21:1576–1577
Erdman DM, Cook CB, Greenlund KJ et al (2002) The impact of outpatient diabetes management on serum lipids in urban African-Americans with type 2 diabetes. Diabetes Care 25:9–15
Keech S, Colquehoun D, Best J et al (2003) Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose: results from the LIPID trial. Diabetes Care 26:2713–2721
Herman WH, Hoerger TJ, Brandle M et al (2005) The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 142:323–332
American Diabetes Association, NIH/NIDDK (2002) The prevention or delay of type 2 diabetes. Diabetes Care 25:742–749
Acknowledgements
This work was supported in part by NIH and NCRR awards DK07298, DK062668, RR017643, DK066204, RR00039 and RR025008, and VA HSR&D awards SHP 08-144 and IIR 07-138. We thank J. Caudle and C. Tsui (systems and database support), and J. Kaufman, E. Osinski, J. Irving, R. Varughese and L. Pinckney (research staff support) for their assistance. This work was presented in part at the national meeting of the American Diabetes Association, June 2008.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
Impact of time after meals and time of day on GCTplasma AROC (PDF 20 kb)
ESM Fig. 1
Study recruitment process (PDF 10 kb)
Rights and permissions
About this article
Cite this article
Phillips, L.S., Ziemer, D.C., Kolm, P. et al. Glucose challenge test screening for prediabetes and undiagnosed diabetes. Diabetologia 52, 1798–1807 (2009). https://doi.org/10.1007/s00125-009-1407-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-009-1407-7